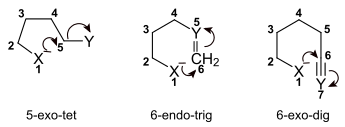Baldwin rules
As Baldwin rules referred to the collection of rules that predict the kinetic preference of chemical reactions that allow with cyclic transition states, mostly cyclization reactions. They were named after their discoverer Jack Baldwin .
nomenclature
The Baldwin rules use a special terminology in which a ring closure is characterized by three parameters:
- The size of the smallest newly formed ring is given in Arabic numerals.
- The position of the split bond relative to the newly formed ring is indicated by the designations exo or endo . Here, exo means that the broken bond points away from the smallest ring formed, and endo that it lies in the ring.
- The geometry of the atom which forms the ring closure is indicated by the abbreviations dig , trig and tet . This depends on the hybridization of the atom: If an sp-hybridized center is attacked, its binding partners are arranged dig onally (i.e. linearly). In one sp 2 -hybridized center its binding partner are trig onal-planar to each other while in a sp 3 -hybridized center tet are arranged raedrisch.
For the nomenclature of the ring closure mechanism, the above-mentioned features - separated from one another by hyphens - are named in the order given.
Preferred and Disadvantaged Mechanisms
| exo | endo | |||||
| dig | trig | tet | dig | trig | tet | |
| 3 | X | ✓ | ✓ | ✓ | X | X |
| 4th | X | ✓ | ✓ | ✓ | X | X |
| 5 | ✓ | ✓ | ✓ | ✓ | X | X |
| 6th | ✓ | ✓ | ✓ | ✓ | ✓ | X |
| 7th | ✓ | ✓ | ✓ | ✓ | ✓ | X |
Baldwin's rules can be used to assess whether it is likely that a particular mechanism will lead to a ring closure. It should be noted that these are not absolute statements, therefore one does not subdivide into “permitted” and “prohibited”, but into “preferred” and “disadvantaged” reactions. However, the rules are only applicable if the nucleophilic reaction center can also attack at the preferred angle of attack: for tet centers the optimal angle is 180 ° (analogous to the Walden reversal ), for trig centers around 107 ° (see also Bürgi-Dunitz Trajectory ) and for dig centers 120 °. If this angle of attack is not accessible due to the existing molecular geometry, no ring closure will occur, even if the reaction according to Baldwin should actually take place preferentially.
The Baldwin rules are based on the principle that all those ring-closing mechanisms take place at a disadvantage, for the process of which a strong distortion of the bond angles and lengths in the molecule would be necessary. In particular, these are:
- 3-exo-dig and 4-exo-dig
- 3-endo-trig , 4-endo-trig and 5-endo-trig
5-endo-trig reactions may appear conclusive when drawn, but they are not plausible and therefore do not take place.
Endo-tet reactions do not lead to ring closures. Even so, they can be predicted by the Baldwin rules. Disadvantaged are:
- 5-endo-tet and 6-endo-tet
The table on the right summarizes the Baldwin rules for three to seven rings; reaction mechanisms marked in green run preferentially, while those marked in red are disadvantaged. With their help, it is possible to predict the preferred product if, as in the example opposite, several reaction paths are conceivable.
The following points should also be observed:
- Baldwin's rules predict kinetic probabilities, not thermodynamics (e.g. equilibria). In the case of preferred reactions, the reverse reaction is also kinetically preferred. In the same way, thermodynamically favored reactions can take place after or instead.
- Cations and molecules with elements of the 3rd period often do not obey Baldwin's rules.
- Reactions that appear to contradict Baldwin's rules may have come about through intermolecular mechanisms.
- Nucleophilic sp 2 centers, e.g. B. enolates, must be considered separately because the orbital symmetry of π orbitals is different.
literature
- JE Baldwin: Rules for Ring Closure . In: J. Chem. Soc., Chem. Commun. No. 18, 1976, pp. 734-736. doi : 10.1039 / C39760000734 ; PDF (280 kB) .
- JE Baldwin, J. Cutting et al: 5-Endo-Trigonal Reactions: a Disfavored Ring Closure . In: J. Chem. Soc., Chem. Commun. No. 18, 1976, pp. 736-738. doi : 10.1039 / C39760000736 ; PDF (220 kB) .
- JE Baldwin, RC Thomas et al .: Rules for ring closure: ring formation by conjugate addition of oxygen nucleophiles . In: J. Org. Chem. Vol. 42, No. 24, 1977, pp. 3846-3852. doi : 10.1021 / jo00444a011 .
- JE Baldwin, LI Kruse: Rules for ring closure. Stereo electronic control in the endocyclic alkylation of ketone enolates . In: J. Chem. Soc., Chem. Commun. No. 7, 1977, pp. 233-235. doi : 10.1039 / C39770000233 .
- JE Baldwin, MJ Lusch et al .: Rules for ring closure: application to intramolecular aldol condensations in polyketonic substrates . In: Tetrahedron . Vol. 38, No. 19, 1982, pp. 2939-2947. doi : 10.1016 / 0040-4020 (82) 85023-0 .
- CD Johnson: Stereo electronic effects in the formation of 5- and 6-membered rings: the role of Baldwin's rules . In: Acc. Chem. Res. Vol. 26, No. 9, 1993, pp. 476-482. doi : 10.1021 / ar00033a004 .
- J. Clayden, N. Greeves et al: Organic Chemistry . In: Chapter 42, pp. 1140ff .; Oxford University Press, USA, 2001, ISBN 0-19-850346-6 .
- FA Carey, RJ Sundberg: Organic Chemistry. A further textbook . P. 157ff .; Verlag Chemie, Weinheim, 1995, ISBN 3-527-29217-9 .