Leuckart-Wallach reaction
The Leuckart-Wallach reaction is a name reaction of organic chemistry and named after the German chemists Rudolf Leuckart and Otto Wallach . It describes the reductive amination of ketones or aldehydes with formic acid . When formaldehyde is used as an alkylating reagent to form methylated amines, this reaction is also called the Eschweiler-Clarke reaction .
Overview reaction
In the Leuckart-Wallach reaction, amines (especially tertiary ) can be produced under simple conditions . Here, an aldehyde or a ketone is reacted with ammonia or a primary or secondary amine and formic acid.
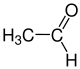
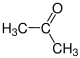
The radicals R 1 to R 4 in the above reaction can be either hydrogen atoms, methyl groups or also aryl groups (e.g. a phenyl group ) or other longer-chain, optionally branched alkyl groups . The following gallery shows the structural formulas of the simplest possible starting materials and formic acid:
Instead of ammonia and formic acid, ammonium formate can also be used, which dissociates into ammonia and formic acid in the course of the reaction when heated . A resonance-stabilized carbenium immonium ion is formed (as is the case with the Mannich reaction, for example ). For the reaction to take place at all, the reaction mixture must be heated to 160–185 ° C.
Reaction mechanism
One possible reaction mechanism is shown below. For the sake of clarity, acetaldehyde is also reacted here with a primary amine (NH 2 CH 3 ).
First, the methylamine ( 2 ) is added to the acetaldehyde ( 1 ) nucleophilically . After condensation , a carbenium ion 3 is formed with two mesomeric limit formulas . A cyclic transition state 4 is then formed with the aid of formic acid . In two steps, this is then first decarboxylated , then deprotonated in order to form the secondary amine 5 .
application
This reaction is mainly used for the synthesis of tertiary amines. In addition, amines with reduction-sensitive groups can be alkylated.
Individual evidence
- ^ Rudolf Leuckart: About a new way of forming tribenzylamine . In: Reports of the German Chemical Society . tape 18 , no. 2 , 1885, p. 2341-2344 , doi : 10.1002 / cber.188501802113 .
- ↑ Peter La Roche deBenneville, Jane Horrocks Macartney: The Behavior of Aliphatic aldehyde in the Leuckart-Wallach reaction . In: Journal of the American Chemical Society . tape 72 , no. 7 , 1950, pp. 3073-3075 , doi : 10.1021 / ja01163a074 .
- ↑ Otto Wallach: On the knowledge of terpenes and essential oils . Twenty-second Treatise. In: Justus Liebig's Annals of Chemistry . tape 272 , no. 1 , 1893, p. 99-122 , doi : 10.1002 / jlac.18932720103 .
- ^ Maurice Lee Moore: The Leuckart Reaction . In: Roger Adams et al. (Ed.): Organic Reactions . tape 5 . John Wiley & Sons , New York 1949, ISBN 978-0-471-26418-7 , Chapter 7, pp. 301-330 , doi : 10.1002 / 0471264180.or005.07 ( 4th print, 1960 [PDF] review article).
- ^ Entry on Leuckart reactions. In: Römpp Online . Georg Thieme Verlag, accessed on February 28, 2014.
- ↑ a b T. Laue, A. Plagens ,: Name and catchword reactions of organic chemistry . Teubner Verlag, Wiesbaden 2006, ISBN 978-3-8351-0091-6 , p. 215-216 .
- ↑ a b c W. Uhl, A. Kyriatsoulis: Name and keyword reactions in organic chemistry . Friedr. Vieweg & Sohn, Braunschweig 1984, ISBN 3-528-03581-1 , pp. 102-104 .