Fragmentation (mass spectrometry)
In mass spectrometry, the fragmentation of molecules occurs during the ionization of the substance to be analyzed. As with electron impact ionization, it can be wanted in order to obtain data for structure elucidation. The main fragmentation reactions are listed below.
Alpha cleavage
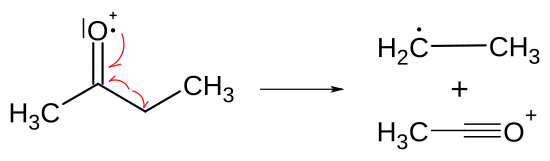
Heteroatoms promote the cleavage of the alpha bond with them. Due to an electron impact, a heteroatom loses an electron from a free, non-binding electron pair and becomes a radical . After the ionization of the hetero atom , an electron moves from the sigma bond of a carbon atom directly adjacent to the hetero atom (the alpha carbon atom) to the hetero atom and, together with the individual electron of the hetero atom, forms a bond between the alpha carbon atom and Heteroatom. This creates a second bond between the heteroatom and the carbon atom and the single electron of the heteroatom is paired again. As a result, the original bond of the alpha carbon atom is broken and its binding partner in turn becomes a radical.
- The course of the alpha split is shown. The single electron of the oxygen forms a triple bond together with a binding electron to the carbon, the bond to the carbon is thereby broken; the ethyl group (top right) becomes a radical (neural particle).
Compounds with heteroatoms that are affected by this rule include amines , alcohols , ethers , thiols , sulfides and halides. Here is the decay-directing effect of
The fragments of amines in the spectrum are extremely intense and easy to recognize, while the fragments of iodides are only very weak. This is related to the ability of the heteroatom to stabilize the positive charge. Since heteroatoms have a high electronegativity , stabilizing a positive charge presents certain difficulties. As a result, homolytic cleavage can best be caused by heteroatoms whose electronegativity is similar to that of carbon - this results in the strong peaks with nitrogen, whose electronegativity is most similar to that of carbon.
With several successive fragmentation reactions, an alpha cleavage takes place only once, since a homolytic cleavage in the cationic product of an alpha cleavage is very energy-consuming. Only a few exceptions are known in the literature.
Benzyl-allyl cleavage
Aromatics that have double bonds lead to alpha cleavages in a similar way to heteroatoms. Benzyl cleavages are more pronounced than allyl cleavages , since the energy gain is greater with the former.
- The course of the benzyl cleavage of butylbenzene is shown. This is the main ion with m / z 91 (center) that dominates the mass spectrum and arises from the loss of a propyl radical. The high intensity in the spectrum results from the great stability of the ion. In the case of the molecules in the first row and second row on the right, both the charge and the radical are distributed over the ring by double bonds and are thus better stabilized.
If a double bond is present in a molecule, it can be assumed that the charge is localized on this. The formation of an allyl ion by homolysis or an allyl radical by heterolysis is then most likely. Theoretically, the resulting mass could be used to find the position of the double bond in open-chain molecules. Unfortunately, this is not possible in practice, since the fragmentation reaction is preceded by numerous isomerization reactions. These isomerizations can be viewed as hydrogen rearrangements. Such isomerizations are less common in alicyclic compounds . In contrast, the cleavage in aromatics gives clear results, since these are very stable. The cleavage of the benzyl bond is greatly favored, thereby generating the benzyl ion. This appears as an intense signal at m / z 91 for almost all compounds that contain benzyl.
Retro-Diels-Alder reaction
In the retro-Diels-Alder reaction, a six-membered ring with a double bond is decycled (split twice) and an ene and a diene component are obtained . The diene is the preferred charge carrier. The retro-Diels-Alder reaction can take place both in the molecular ion and in a fragment. If there is no nitrogen present, the reaction yields even-numbered fragments of even-numbered molecular ions, which is noticeable among the otherwise mostly odd-numbered fragments. The decyclized ring can, but does not have to, contain heteroatoms. The reaction is very sensitive even to minor structural changes; while a particular compound forms a strong peak in the mass spectrum, a very similar compound may almost not be seen in the mass spectrum.
- The retro-Diels-Alder reaction is shown. Homolysis takes place at the top and in the middle, while heterolysis takes place at the bottom.
In the Diels-Alder reaction, the initial ionization always takes place on the double bond. In the following two reaction courses are possible in which the charge either remains in the original position or is shifted.
The retro Diels-Alder reaction in mass spectrometry was not with the equally as retro-Diels-Alder reaction designated cycloreversion be confused. The latter is a concerted reaction of non-ionized Diels – Alder adducts.
McLafferty rearrangement
In the McLafferty rearrangement , a six-membered transition state transfers a gamma hydrogen atom to an at least doubly bonded atom. The alpha bond is split, a neutral particle is released and the double bond is shifted. The double bond required for the McLafferty rearrangement does not have to be present in the original molecule, but can also be formed in the course of a fragmentation reaction, e.g. B. by an alpha split. The acceptor molecule for the proton can be a C = O, C = N or a C = C bond, among other things. If there is no H atom on the gamma C atom, the reaction does not take place. The McLafferty rearrangement is an analogous reaction to the Norrish Type II reaction .
Neutral loss
In order to identify a mass spectrum, it is usually necessary to use the M + . -Identify peak. The following table can help, it contains some neutral (uncharged) fragments that may be different. a. can split off from the molecular ion. In principle, the table can also be applied to secondary fragments, but it is most useful for splitting off the molecular ion, since otherwise a mass difference, which can also be found in this table, does not necessarily have to be due to a loss of neutrality.
In this way, assumptions can also be made about the structures originally present in the molecule. It should also be noted that several neutral losses can follow one another.
| Dimensions | molecule |
|---|---|
| M-1 | H . |
| M-15 | CH 3 . |
| M-16 | NH 2 . |
| M-17 | OH . , NH 3 |
| M-18 | H 2 O |
| M-19 | F . |
| M-20 | HF |
| M-26 | HCCH, CN . |
| M-27 | HCN, H 2 C = C . H |
| M-28 | CO, H 2 C = CH 2 |
| M-29 | CH 3 C . H 2 , HCO . |
| M-30 | H 2 CO |
| M-31 | CH 3 O . |
| M-32 | CH 3 OH, S |
| M-33 | HS . |
| M-35 | Cl . |
| M-36 | HCl |
| M-42 | H 2 C = C = O, H2C = CH-CH 3 |
| M-43 | CH 3 C . O, C 3 H 7 . |
| M-44 | CO 2 |
| M-45 | CH 3 CH 2 O . , . CO 2 H |
| M-46 | NO 2 |
| M-57 | CH 3 CH 2 C . O, . C 4 H 9 |
| M-77 | C 6 H 5 . |
| M-79 | Br . |
| M-80 | HBr |
| M-91 | C 6 H 5 C . H 2 |
| M-127 | I . |
Individual evidence
- ↑ Manfred Hesse, Herbert Meier, Bernd Zeeh: Spectroscopic methods in organic chemistry. 7th edition. Georg Thieme, Stuttgart 1995, ISBN 3-13-576105-3 , p. 250.
- ↑ Josef Seibl, Walter Wolfgang: mass spectrometry study book for chemistry students after the intermediate diploma. 2nd Edition. Akademische Verlagsgesellschaft, Frankfurt am Main 1970, ISBN 3-400-00000-0 , pp. 64–67.
- ^ A b c R. Martin Smith: Understanding mass spectra: a basic approach. 2nd Edition. John Wiley & Sons, New Jersey 2004, ISBN 0-471-42949-X , pp. 164-166.
- ↑ a b Manfred Hesse, Herbert Meier, Bernd Zeeh: Spectroscopic methods in organic chemistry. 7th edition. Georg Thieme, Stuttgart 1995, ISBN 3-13-576105-3 .
- ↑ Josef Seibl, Walter Wolfgang: mass spectrometry study book for chemistry students after the intermediate diploma. 2nd Edition. Akademische Verlagsgesellschaft, Frankfurt am Main 1970, ISBN 3-400-00000-0 , pp. 56–59.
- ↑ Josef Seibl, Walter Wolfgang: mass spectrometry study book for chemistry students after the intermediate diploma. 2nd Edition. Akademische Verlagsgesellschaft, Frankfurt am Main 1970, ISBN 3-400-00000-0 .