Tris (trimethylsilyl) phosphine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tris (trimethylsilyl) phosphine | |||||||||||||||
| other names |
Tris (trimethylsilyl) phosphine |
|||||||||||||||
| Molecular formula | C 9 H 27 PSi 3 | |||||||||||||||
| Brief description |
colorless solid or colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 250.54 g mol −1 | |||||||||||||||
| Physical state |
solid or liquid |
|||||||||||||||
| density |
0.86 g cm −3 |
|||||||||||||||
| Melting point |
24 ° C |
|||||||||||||||
| boiling point |
102-105 ° C (13 h Pa ) |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tris (trimethylsilyl) phosphine is a tertiary phosphine with the empirical formula C 9 H 27 PSi 3 . It is used in laboratories instead of the gaseous and extremely toxic monophosphine (PH 3 ) for the synthesis of various phosphorus compounds .
At 20 ° C the connection is solid and can self-ignite when exposed to air . It is therefore worked with protective gas . As with many other phosphines, this compound has a very unpleasant odor of putrefaction.
synthesis
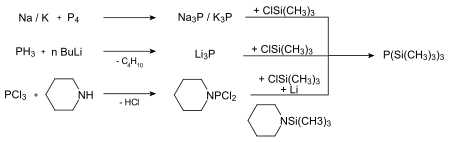
In principle there are three ways of producing tris (trimethylsilyl) phosphane.
The first two synthetic routes are technically feasible, but less suitable for everyday laboratory use.
Niecke and Westermann were able to establish an alternative and easily practicable process in which the protected phosphane is accessible in good yields. It can also be obtained by converting trimethylchlorosilane , phosphorus trichloride and magnesium in tetrahydrofuran .
properties
Tris (trimethylsilyl) phosphane is very often used as a synthetic building block for adding a phosphine group to existing molecules. The trimethylsilyl groups serve only temporarily for protection and increase the nucleophilicity of the phosphorus. The easy splitting off of the protective groups is ensured by the high oxophilicity ( affinity of silicon to oxygen).
For example, tris (trimethylsilyl) phosphine can be used to obtain cyaphide , an isoelectronic phosphorus analog of cyanide . In this reaction, tris (trimethylsilyl) phosphine is converted with acyl halides and subsequent β-elimination to form organyl cyaphid.
Transition metal complexes
Metal carbonyls can serve as starting molecules for larger metal atom clusters . Phosphine compounds have proven to be bridging agents. The attachment of tris (trimethylsilyl) phosphane enables access to PH 3 clusters, which can be converted into clusters with three or more metal atoms , for example by further thermal treatment . Just one example of many is the reaction of dirhenium decacarbonyl with tris (trimethylsilyl) phosphane, which after subsequent methanolysis (conversion of the trimethylsilyl groups into hydrogen) gives Re 2 (CO) 9 PH 3 . Thermal or photochemical further treatment results in Re 2 (µ-H) (µ 3 -PHRe (CO) 5 ) (CO) 8 , in which the phosphorus acts as a µ 3 -P bridge and thus binds three rhenium atoms .
The hydrogen atoms of the PH 3 ligand can also be exchanged for metal carbonyl halides step by step after deprotonation and the μ-H bridges also obtained can be exchanged isolobally with ClAuPPh 3 . In 2001, for example, Re 2 (μ-AuPPh 3 ) (μ 4 -P (Fe (cis-Cp) CO) 2 (μ-CO)) (CO) 8 , a cluster with a μ 4 -P bridge, was synthesized.
If coin metal halides are reacted with tris (trimethylsilyl) phosphane, highly aggregated metal clusters with P 3− bridges are formed. A selected example is the reaction with CuCl, in which the cluster [Cu 96 P 30 {P (SiMe) 3 } 6 (PEt 3 ) 18 ] is formed.
Individual evidence
- ↑ a b c d data sheet tris (trimethylsilyl) phosphane from AlfaAesar, accessed on March 10, 2010 ( PDF )(JavaScript required) .
- ↑ Tris (trimethylsilyl) phosphine data sheet (PDF) from Strem, accessed on December 25, 2012.
- ↑ a b Data sheet Tris (trimethylsilyl) phosphine from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).
- ↑ Niecke, Westermann: A simple process for the production of tris (trimethylsilyl) phosphane , in: Synthesis , 1988 , p. 330; doi : 10.1055 / s-1988-27560 .
- ↑ H. Schumann , L. Rösch: Representation and vibration spectra of trimethylsilyl, trimethylgermyl and trimethylstannyl-tert-butylphosphines , in: Chem. Ber. , 1974 , 107 , pp. 854-868; doi : 10.1002 / cber.19741070312 .
- ^ M. Regitz: Phosphaalkynes: new building blocks in synthetic chemistry , in: Chem. Rev. , 1990 , 90 , pp. 191-213; doi : 10.1021 / cr00099a007 .
- ↑ H.-J. Haupt, O. Krampe, U. Flörke: Representation and molecular structures of oligofunctional dirhenium carbonyl derivatives from dirhenium nonacarbonylphosphane , in: Z. anorg. allg. Chem. , 1996 , 622 , pp. 807-812; doi : 10.1002 / zaac.19966220510 .
- ↑ P. Haferkamp: On the metal expansion of two-core complexes of group 7 on the basis of PH-acidic ligands: synthesis, structures, properties and catalysis ; Dissertation University of Paderborn 2001 .
- ↑ Fenske et al .: New Phosphido-Bridged Copper Clusters , in: Angew. Chem. , 1993 , 105 , pp. 257-261; doi : 10.1002 / anie.19931050214 .
literature
- Louis D. Quin: A Guide to Organophosphorus Chemistry . Wiley-Interscience, 2000, ISBN 0-471-31824-8 .
- Christoph Elschenbroich , A. Salzer: Organometallchemie . Teubner Taschenbücher Chemie, 1990, ISBN 3-519-23501-3 .