Phosphanes
Phosphanes and phosphines denote the same class of compounds, the compounds of which consist of a trivalent phosphorus atom or corresponding phosphorus chains to which hydrogen or organic substituents are bonded. Phosphine is the original name, but it is not IUPAC- compliant (phosphine is reminiscent of the lighter nitrogen-analogous compound class of amines ). In the following only phosphine is used.
structure
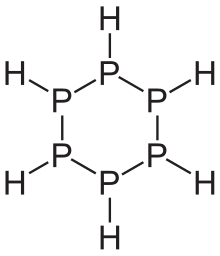
Phosphanes show a variety of structures. Linear phosphines are known up to a chain length of six phosphorus atoms. They have a similar structure to the alkanes , but the phosphorus atom as the fourth substituent each has a pair of electrons. Therefore they have the general empirical formula P n H n + 2 . There are also connections with the sum formula P n H n and P n H n − 2 . As with hydrocarbons , there are also ring-shaped phosphines. However, cage-like structures are particularly pronounced, which relatively often also contain otherwise unusual, because unstable rings of three. All phosphanes - with the exception of monophosphine - have in common that they consist of a framework of phosphorus atoms that are linked by single bonds.
nomenclature
The group of phosphanes, which only consist of hydrogen and phosphorus, are also called phosphorus hydrides . The simplest compound from this group is monophosphane (PH 3 ). Analogous to the boranes , the name of the phosphanes is formed from a Greek numerical word corresponding to the number of phosphorus atoms , the designation phosphane and an Arabic number in brackets for the number of hydrogen atoms, e.g. triphosphane (5) for P 3 H 5 or heptaphosphane (3) for P 7 H 3 . At this point it should be noted that the corresponding pentavalent compound PH 5 , the so-called phosphorane, does not exist. In the case of organic derivatives , too, a corresponding five-bond structure is only rarely represented (e.g. in PPh 5 ).
Organic derivatives of monophosphine (PH 3 ) are formally formed by exchanging hydrogen (H) for organic residues - generally referred to as R. The functional group derived from the phosphines is called the phosphino group. Depending on whether phosphines have one (PH 2 R), two (PHR 2 ) or three (PR 3 ) organic radicals, one speaks of primary, secondary or tertiary phosphines , as with amines . Phosphines that carry short alkyl groups , such as trimethylphosphine [(H 3 C) 3 P], are liquids with an unpleasant smell. They are very reactive and tend to self-ignite. Triarylphosphines such as (H 5 C 6 ) 3 P are solid substances that have weak basic properties. They easily react to form phosphonium salts , taking up another organic residue . Organophosphorus compounds that contain three POC binding units are called phosphites (formula: P (OR) 3 ).
properties
Phosphorus Hydrogen Compounds
Phosphines are extremely toxic substances. Monophosphine (PH 3 ) is gaseous, diphosphine (P 2 H 4 ) is a colorless liquid which can spontaneously ignite in air at room temperature, the higher ones are solid. Phosphanes are very reactive. Very pure monophosphine PH 3 does not ignite by itself. Due to the presence of higher phosphines (especially diphosphine ), self-ignition must always be expected when using commercially available monophosphine or prepared in a laboratory. These phosphorus compounds have an extremely pervasive garlic-like odor, which is noticeable even in the smallest concentrations.
Organic phosphines
Organic phosphines with short alkyl groups are liquid and very sensitive to air, in some cases also sensitive to light (for example t -Bu 3 P, because of the possibility of α- H abstraction). They too have a characteristic pungent odor that is vaguely reminiscent of garlic.
The basicity of the phosphine is significantly increased by alkyl groups, and indeed to a much greater extent than is the case with ammonia. The pK b value of phosphane is 27 and that of triethylphosphine is 5.31. In comparison, the pK b values from ammonia (4.75) to triethylamine (3.25) decrease only slightly.
Triarylphosphines, on the other hand, are solid and much more stable. They can be stored in the solid state without any problems and are only sensitive to oxidation in solution. The basic substances are odorless, but commercial products can be contaminated with primary and secondary phosphines that have an odor.
Two compounds are known among the methylchlorophosphines, methyldichlorophosphine CH 3 PCl 2 and dimethylchlorophosphine (CH 3 ) 2 PCl. Both substances are colorless liquids, spontaneously ignitable in air and have a high reactivity. The synthesis can be carried out by reacting a phosphorus vapor - methylene chloride mixture at 350 ° C over a charcoal catalyst derives. Another possibility exists via the Kinnear-Perren reaction of phosphorus trichloride , aluminum chloride and methyl chloride via the complexes [CH 3 PCl 3 ] [AlCl 4 ] or [(CH 3 ) 2 PCl 2 ] [AlCl 4 ] as an intermediate. Because of their high reactivity, a large number of organophosphorus compounds can be produced from methylchlorophosphines.
Extraction / representation
Monophosphine
There are numerous ways to represent monophosphine , e.g. B. disproportionate white phosphorus (P 4 ) in an alkaline medium to phosphine and phosphinic acid :
Analogous to the production of ammonia in the Haber-Bosch process , a synthesis from the elements can also take place:
Calcium phosphide or aluminum phosphide is allowed to react with water in the laboratory :
In addition to monophosphine, some diphosphine is also formed.
Finally, monophosphane can also be obtained from phosphonium iodide and potassium hydroxide :
Organic phosphines
Phosphines can be obtained from monophosphine or phosphorus trichloride . In substitution reactions , hydrogen or chlorine are replaced by organic residues.
Monophosphane can be reacted with alkyl halides or olefins :
- Monophosphine reacts with methyl chloride to form trimethylphosphine and hydrogen chloride .
Starting from phosphorus trichloride, Grignard or organolithium compounds are used :
- Phosphorus trichloride reacts with phenylmagnesium bromide to form triphenylphosphine and magnesium bromide / magnesium chloride .
Instead of PCl 3 , the use of phosphites , e.g. B. P (OPh) 3 or P (OMe) 3 (where Ph = phenyl, -C 6 H 5 and Me = methyl, -CH 3 ) are advantageous, as these are not so prone to side reactions (formation of organic diphosphanes R 2 P-PR 2 ) and are easier to use.
Another possibility for representation is the hydrophosphorylation of double and triple bonds. This can proceed with a base catalysis or be initiated photolytically with a radical starter (e.g. azobis (isobutyronitrile) , AIBN):
use
Phosphanes are of outstanding importance as ligands in homogeneous catalysis . They stabilize a large number of catalysts and, thanks to their structural diversity, enable the catalyst properties to be adjusted. The enantiomerically pure preparation of many organic compounds (especially pharmaceuticals and their starting compounds) has only been made possible through the use of chiral phosphanes . Catalysts that contain phosphine ligands or for olefination reactions are also frequently used in the manufacture of plastics .
Phosphines are also used as reagents in a number of chemical reactions, e.g. B. Mitsunobu reaction , Wittig reaction , etc. Here, the high oxophilicity of phosphorus is used, d. H. the high tendency of a phosphorus atom to form bonds with oxygen atoms (here O = P double bonds).
Phosphines are also semiconductors via organometallic gas phase epitaxy (MOVPE) for the production of phosphorus-containing III-V compounds , e.g. B. doped silicon , GaP , GaAsP and InGaAsP for light emitting diodes and semiconductor lasers as well as ultrafast transistors ( HEMTs ) are used.
Phosphorus hydrogen is used as a rodenticide . Aluminum phosphide and calcium phosphide are introduced into vole exits and form phosphorous hydrogen with moisture. Zinc phosphide is used as feeding bait. Here, phosphine is formed in the stomach.
Monophosphane is used as a fumigant to kill stored pests in storage rooms or containers. What is allowed for conventionally produced goods is not allowed for organically produced products.
Derivatives
Phosphine oxide ( phosphoryl hydride ) O = PH 3 , the oxide of monophosphine, only exists in the form of alkyl and aryl derivatives OPR 3 , OPHR 2 and OPH 2 R. If the three hydrogen atoms in the phosphine oxide are replaced by monovalent radicals (except for hydroxyl groups), see below the phosphoryl compounds are obtained (see e.g. phosphoryl chloride ); if they are replaced one after the other by one to three hydroxyl groups , the result is phosphinic , phosphonic or phosphoric acid .
Tris (trimethylsilyl) phosphine is often used as an easier-to-use substitute for the phosphine as a synthetic building block.
See also
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , pp. 757-758.
Individual evidence
- ↑ Michael Binnewies, Manfred Jäckel, Helge Willner, Geoff Rayner-Canham: Allgemeine und Anorganische Chemie. Spectrum Akademischer Verlag, Heidelberg et al. 2003, ISBN 3-8274-0208-5 .
- ^ Francis A. Carey, Richard J. Sundberg: Organic Chemistry. A further textbook. 2. corrected reprint. Wiley-VCH, Weinheim 2004, ISBN 3-527-29217-9 .
- ↑ Horst Staendeke, Hans-Jerg Kleiner: Methylchlorphosphane and follow-on products. In: Angewandte Chemie . 85th vol., 1973, No. 22, pp. 973-978.
- ↑ Georg Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. Volume 1. 2nd edition. Academic Press, New York et al. 1963, pp. 525-530.
- ↑ Phosphine on Galab's website , accessed April 28, 2018.