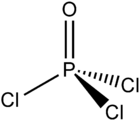
Phosphorus oxychloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Phosphorus oxychloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | POCl 3 | |||||||||||||||
| Brief description |
colorless to yellowish viscous liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 153.33 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.68 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
1.25 ° C |
|||||||||||||||
| boiling point |
105.8 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
violent decomposition with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phosphorus oxychloride is a poisonous liquid that smokes heavily in the air and is formally the trichloride of phosphoric acid .
Extraction and presentation
On a laboratory scale
On a laboratory scale, phosphorus oxychloride can be produced by introducing sulfur dioxide into phosphorus pentachloride .
The slightly yellowish crude product of the reaction - a mixture of POCl 3 and thionyl chloride (SOCl 2 ) - is separated by fractional distillation.
Further display options are:
- the reaction of oxalic acid with phosphorus pentachloride
- the oxidation of phosphorus trichloride with potassium chlorate
- and the reaction of phosphorus pentachloride with phosphorus pentoxide
- .
Industrial process
The industrial production of phosphorus oxychloride takes place in different ways. Since phosphorus oxychloride is used as an important inorganic raw material in numerous cost-critical manufacturing processes, the production costs play a decisive role in the process selection. The focus is on technically mature processes that provide the end product in an appropriately high quality and generate little waste. Particularly preferred are processes that allow the use of inexpensive raw materials or that can further utilize the by-products of other manufacturing processes.
An example of this strategy is the production of phosphorus oxychloride from sulfur dioxide (SO 2 ), which occurs as waste gas in many processes , gaseous chlorine (Cl 2 ) and phosphorus trichloride (PCl 3 ):
Optimum results are achieved when the process is carried out continuously in a continuous stirred tank reactor or loop reactor.
Phosphorus oxychloride can also be obtained from the phosphine-containing waste gases from hypophosphite production:
The process initially produces phosphorus trichloride (PCl 3 ), which is then oxidized with oxygen to form the end product.
In the production of certain heterocyclic compounds - important intermediates and the like. a. in the production of pesticides - phosphorus oxychloride is a by-product. By separating off the reusable oxychloride, the production costs of the heterocycle synthesis can be reduced significantly.
A particularly pure phosphorus oxychloride provides the direct oxidation of phosphorus trichloride (PCl 3 ) with oxygen (O 2 ) in the liquid phase. The process is particularly used when the production of phosphorus trichloride, another important basic chemical in the chemical industry, is sought.
There is also a continuous variant for this process. The reaction is strongly exothermic with a molar heat of reaction of −279.5 kJ mol −1 .
In the fine chemical industry, the phosphorus trichloride oxidation is usually carried out discontinuously in a stirred tank reactor . However, the disadvantage of this process control variant is the unfavorable volume yield of the reaction and the relatively complex separation of the oxychloride from the starting material. From the product specification, the effort that was made in separating the end product and also the quality of the production plant can often be read off.
There is a method which addresses the problems described above. Instead of phosphorus trichloride , white phosphorus is used as the starting material for the reaction. The phosphorus is first oxidized with oxygen and the phosphorus oxide is then chlorinated with elemental chlorine . The reaction is carried out in the liquid phase. The unpurified oxychloride is used as the reaction medium and solvent - this is also an interesting feature of the manufacturing process.
Phosphoryl chloride can also be produced by directly burning phosphorus in an oxygen-chlorine flame. However, the process delivers a very complex mixture of products and is extremely difficult to control technically.
A further development of the methodology described above by Knappsack-Griesheim AG is presented in the Japanese patent published in 1994 by Nippon Sōda . The Japanese process is particularly notable for its improved process control, which allows precise control of the chlorination.
In the older patent literature numerous other processes for the production of phosphorus oxychloride are described, but these are no longer used today.
properties
Physical Properties
POCl 3 melts at 1.25 ° C and is a colorless, clear, very toxic , light-refractive liquid that is sensitive to hydrolysis and fumes heavily in the air at room temperature and boils at 105.8 ° C. Liquid phosphorus oxychloride at 25 ° C, a density of 1.645 g / cm 3 . In the gas state, the density is 5.3 (air = 1). The vapor pressure over liquid phosphorus oxychloride is 37 hPa or 139 hPa at 20 ° C or 50 ° C. According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar , T in K) with A = 4.28166, B = 1445.959 and C = −40.119 in the temperature range from 275 to 378.3 K. The specific conductivity of the liquid oxychloride is 2 · 10 −8 Ohm −1 · cm - 1 and is based on the low self-dissociation of the oxychloride in POCl 2 + and POCl 4 - . In practice, the residual moisture of 0.0001 mol / l, which is always present even in extremely purified POCl 3, contributes to the conductivity , which leads to the formation of H 3 O + Cl - .
Liquid phosphorus oxychloride is a very good non-aqueous solvent in which metal chlorides - with the exception of alkali chlorides - dissolve to form highly conductive solutions. By means of a conductivity measurement , impurities in pure phosphorus oxychloride can be detected very conveniently as sum parameters .
Complex formation is observed with transition metals .
Material compatibility
Phosphorus oxychloride is u. a. extremely corrosive and material attacking due to its chloride content . Among the stainless steels , austenitic chromium-nickel steel and the stainless steel grades 18–8 and 17-12-3 are only attacked moderately quickly by phosphorus oxychloride. The materials mentioned are therefore suitable for the construction of storage containers and tanks that are used for the temporary storage of phosphorus oxychloride or its transport. Lead , nickel and monel have a higher resistance . Due to the risk of corrosion , glass or glass- reinforced stainless steel and the like is preferred for the construction of lines and for the construction of reactors and long-term storage containers for phosphorus oxychloride . U. plastic-coated stainless steel used. The exclusive use of plastic is problematic because of the moisture and gas permeability of practically all plastics and the risk of the product being contaminated by accompanying plastic materials. Among the plastics, the following types of plastics have limited compatibility with phosphorus oxychloride at temperatures up to approx. 30 ° C:
- High density polyethylene (HDPE)
- Polypropylene (PP)
- Polyvinylidene fluoride (PVDF)
- Ethylene tetrafluoroethylene copolymer (ETFE)
Only ETFE is recommended for higher operating temperatures.
In the case of fluoroplastic-coated containers, it should be noted that the coating consists of sintered or fused polymer powder, is not necessarily pore-free and can contain binding agents.
In general, one should take into account that plastics are technical products with a non-uniform composition. Even plastics with the same abbreviation often differ considerably in their properties. Furthermore, some abbreviations are used as trade names and denote an entire product group. In this sense z. For example, terms such as Teflon and PTFE are often used. In the absence of reliable material compatibility data, one should refrain from using a certain material or carry out a specific usage test. To draw conclusions from the behavior of the material under subjectively similar appearing operating conditions is extremely risky in terms of safety.
Chemical properties
Phosphorus oxychloride reacts violently with water , metal , bases , acetone (presumably all ketones ), alcohols , amines , phenols , strongly oxidizing and organic substances.
Solvents to be used for reactions with phosphorus oxychloride must therefore be anhydrous. Dry solvents can be removed by drying e.g. B. can be produced easily and conveniently using molecular sieves . Solvents containing more water should be predried with sodium sulfate beforehand. An explosive reaction can occur on contact with the solvents dimethylformamide or dimethyl sulfoxide .
use
Phosphorus oxychloride is an important raw material for the production of phosphoric acid esters ( alkyl and aryl phosphites). The synthesis is carried out by reacting phosphorus oxychloride with alcohols , phenols or epoxides and provides economically important products such as
- Plasticizers for PVC and other plastics
- Flame retardants
- Fuel additives
- Hydraulic oils and hydraulic oil additives
- Extractant for uranium processing and metal extraction
- insecticides
The use of phosphorus oxychloride for the production of organophosphorus insecticides is closely related to its use in the production of organophosphorus nerve gases belonging to the same family of substances .
Phosphorus oxychloride is still called
- Chlorinating agents for the production of carboxylic acid chlorides and acid anhydrides
- Catalyst in the manufacture of triphenylmethane dyes
- Reagent for the formation of the Vilsmeier-Haack complex
- non-aqueous solvent
as well as
- Auxiliary in the production of optical glass fibers
- n-type dopants in semiconductor and solar cell production
used.
Production quantities and manufacturers
Phosphorus oxychloride is one of the indispensable inorganic basic chemicals in the chemical industry. Worldwide annual production in 2002 was an estimated 200,000 t. About 150,000 tons / year of the production capacity is in the OECD countries and 50,000 tons / year in third countries. In 1995 the production capacities were about 39,900 tons in the USA, 100,000 tons in Western Europe and 30,000 tons in Japan. In 2004 there were four manufacturers of phosphoryl trichloride in Western Europe. Three of them had production facilities in Germany. The main production areas are Europe, the USA and Japan. China, India and Australia are also important producing countries. Typical production facilities have annual production capacities in the range of 5,000 to 10,000 tons.
A large part of the phosphorus oxychloride is processed directly into secondary products. The production of phosphorus oxychloride is therefore often associated with the manufacture of important secondary products, e.g. B. with the production of plastic additives , flame retardants , pesticides , lubricants and so-called functional liquids, networked.
Important phosphorus oxychloride manufacturers are Lanxess (D), BASF, Akzo Nobel, Monsanto, Rhodia, Syngenta, Taixing Shenlong Chemical, Fu Tong Chemical, Jiangsu Jibao Technology, Wynca, Xuzhou JianPing Chemical and others.
Quality and form of delivery
The phosphorus oxychloride, which is produced by one of the synthesis processes described above, is initially obtained as a more or less highly contaminated crude product. The impurity profile of the raw product is determined by the manufacturing method, the impurity profile of the raw materials and, last but not least, the condition of the production facility. Modern production plants consistently deliver a very high quality and pure raw product. The crude product is further purified by fractional distillation, which is supplemented by solid phase absorption if necessary . In the distillation, liquid and gaseous reaction by-products as well as raw material residues - z. B. phosphorus trichloride (PCl 3 ) - separated. The effort involved in purifying the crude product is of course subject to strict economic controls.
Other factors that have a decisive influence on product purity are the handling of the product after purification, the product packaging, the storage of the product at the place of manufacture and the transport, storage and handling of the oxychloride by the intermediate trade and end user.
A large part of the industrially produced phosphorus oxychloride is consumed in subsequent processes immediately after production. Phosphorus oxychloride is marketed with a specified purity of 98–99.9999%. For phosphorus oxychloride, which comes from modern large-scale production plants, basic product purities> 99.9% are typical. Phosphorus trichloride (PCl 3 ) is usually mentioned as the main impurity . The level of metal contamination, which is important for some applications, is usually very low in freshly produced phosphorus oxychloride, since even trace concentrations of transition metals inhibit the POCl 3 production process and are therefore carefully avoided from the outset. As is usual with chemical products, the total purity stated in the product specification mostly relates to the total amount of impurities recorded in the product analysis. Furthermore, when drawing up product specifications, marketing aspects play an important role. So it is e.g. B. It is common practice to offer products of high purity to different target markets, each with an adapted specification.
A large part of the commercially available phosphorus oxychloride has a specified minimum purity of> 98.5% to> 99.9% and reaches the end user either directly or via chemical intermediaries. The pack size depends on the intended use. Small quantities are offered by the laboratory trade in plastic bottles with a content of up to 0.6 kg. Larger product quantities are delivered to the buyer in plastic canisters with a capacity of 50 kg or in drums made of plastic or plastic-coated steel with a capacity of up to 300 kg. Tank and tank wagons are used for even larger quantities of product.
For special applications, e.g. B. for the production of glass fiber , semiconductors or solar cells , phosphorus oxychloride is offered with a total purity of> 99.999%. The so-called high-purity and ultra-pure phosphorus oxychloride is mostly identical to the standard product from selected major manufacturers. Since the analysis of phosphorus oxychloride is extremely error-prone, the original manufacturer's batch analysis is usually used.
With the solid-phase absorption process described by Mykrolis Corporation, high-purity phosphorus oxychloride can be produced in an elegant manner, quasi at the point of use. It is particularly advantageous here that at least some of the impurities that got into the product during storage are captured and removed.
The delivery of the end users of high-purity phosphorus oxychloride takes place via the laboratory trade or via specialized intermediaries. Only glass ampoules, glass bottles or glass-reinforced stainless steel containers are suitable for packaging. Plastic containers and plastic-coated containers are not suitable (see below).
Trade restrictions
Phosphorus oxychloride can be used to make toxins that can be used in weapons of mass destruction . The export of phosphorus oxychloride is therefore subject to strict controls and authorization requirements in all industrialized countries.
safety instructions
Phosphorus oxychloride reacts directly and strongly exothermically with moist air or with water . The hydrolysis reaction leads to the formation of highly corrosive mist containing phosphorus and hydrochloric acid, which can also contain vaporous phosphorus oxychloride. If small amounts of phosphorus oxychloride are leaked or spilled, a certain protection against poisoning and chemical burns results from the fact that phosphorus oxychloride evaporates slowly due to its low vapor pressure, the vapors are significantly heavier than air and evaporated phosphorus oxychloride is hydrolyzed very quickly in moist air. In the event of spillage or leakage in closed rooms, on the other hand, it is essential to leave the room immediately.
See also
literature
- ADF Toy: Phosphorus Chemistry in Every Day Living , American Chemical Society, Washington 1976
- E. Fluck, K. Maas (Hersg): Topics on the chemistry of phosphorus , Dr. Alfred Hüttig Verlag GmbH, Heidelberg 1973
- Ullmann's Encyclopedia of Technical Chemistry , 6th ed., Vol 26, p. 193, Phosphorous Compound, Inorganic , Verlag Wiley - VCH, Weinheim, 2002
Web links
- Safety data sheet
Individual evidence
- ↑ a b c d e f g h i j k Entry on phosphoryl trichloride in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on Phosphoryl trichloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Data sheet phosphoryl chloride (PDF) from Merck , accessed on February 3, 2017.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 10025-87-3 or phosphorus oxychloride ), accessed on November 2, 2015.
- ↑ U.S. Patent US5498400, Great Lakes Chemical Corp.
- ↑ U.S. Patent US6685904, Occicdental Chemical Corp.
- ↑ patent EP0900762, DSM Fine Chemicals Austria GmbH.
- ↑ DE19730224, Bayer AG.
- ^ A b G. Bettermann, W. Krause, G. Ries, T. Hofmann: Phosphorus Compounds, Inorganic , in: Ullmanns Enzyklopädie der Technischen Chemie , Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim 2012; doi : 10.1002 / 14356007.a19_527 .
- ↑ U.S. Patent US4183905, Mobil Oil Corp.
- ↑ a b Patent application DE1194382 : Process for the production of phosphorus oxychloride and / or higher phosphorus-chlorine-oxygen compounds of the general formula POCl. Registered on July 12, 1962 , published on June 10, 1965 , applicant: Knapsack-Griesheim AG, inventors: Mueller-Schiedmayer, Heinz Harnisch, Joseph Cremer.
- ↑ Patent DE801513 : Process for the production of phosphorus oxychloride. Registered on October 2, 1948 , published on January 11, 1951 , applicant: BASF, inventor: Alfons Janson.
- ↑ JP6122509, Nippon Soda Corp.
- ↑ Stull, DR: Vapor Pressure of Pure Substances Organic Compounds in Ind. Eng. Chem. 39 (1947) 517-540.
- ↑ OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Phosphoryl trichloride , accessed on September 23, 2015.
- ↑ 360 Market Updates: Global Phosphorus Trichloride Industry Production Sales And Consumption Status And Prospects Professional Market Research Report 2017 2022 - 360 Market Updates , accessed on October 12, 2017.
- ↑ patent WO2005092790, Mykrolis Corporation.











