Metominostrobin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
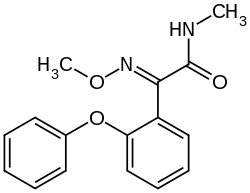
|
||||||||||||||||
| Structural formula of ( E ) -metominostrobin | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Metominostrobin | |||||||||||||||
| other names |
Methyl-α-methoxyimino- N -methyl-2-phenoxyphenylacetamide |
|||||||||||||||
| Molecular formula | C 16 H 16 N 2 O 3 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 284.31 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.28 g cm −3 (bulk density) |
|||||||||||||||
| Melting point |
87-89 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Metominostrobin is a chemical compound from the group of methoxyiminoacetamides and strobilurins , which is used to control fungi in agriculture.
properties
Metominostrobin is a white solid that is poorly soluble in water. Because of the asymmetrically substituted oxime double bond, metominostrobin occurs as an E / Z isomer pair . Since the E isomer is 5 to 20 times more effective than the Z isomer, the E isomer is predominantly used.
use
Metominostrobin is used as a fungicide in rice against the fungus Magnaporthe grisea and is approved in Asian countries. The connection was discovered in 1998 by the Shionogi company .
Individual evidence
- ↑ a b c d Robert Krieger: Handbook of Pesticide Toxicology, Two-Volume Set: Principles and Agents . Academic Press, 2001, ISBN 0-08-053379-5 , pp. 1203 ( limited preview in Google Book search).
- ↑ a b c data sheet (E) -Metominostrobin from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ a b c d e Entry on Metominostrobin in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on January 31, 2015.