Kresoxim-methyl
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Kresoxim-methyl | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 19 NO 4 | ||||||||||||||||||
| Brief description |
colorless and odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 313.35 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
97-102 ° C ° C |
||||||||||||||||||
| boiling point |
Decomposes at 310 ° C |
||||||||||||||||||
| Vapor pressure |
2.3 · 10 −6 Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Kresoxim-methyl is an active ingredient for plant protection and a chemical compound from the group of strobilurins .
presentation
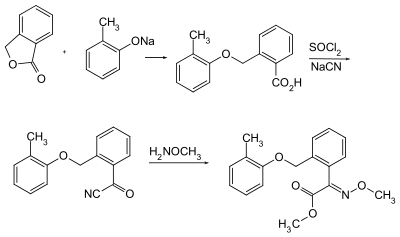
Kresoxim-methyl can be obtained by a multistage reaction starting from phthalide (3 H -isobenzofuran-1-one).
The first step in the synthesis is a nucleophilic attack of a phenolate ion on a lactone and a benzyl aryl ether is formed with opening of the lactone. In the next step, the acid chloride is first produced, which reacts with sodium cyanide to form an α-carbonyl nitrile in the next reaction . In the last step this is reacted with O -methylhydroxylamine and kresoxim-methyl is obtained.
properties
Kresoxim-methyl is a colorless and odorless solid that is insoluble in water.
use
Kresoxim-methyl is used as a fungicide -Wirkstoff in pesticides used. The technical product contains up to 50% of the active ingredient and is a brown solid with a slightly sulphurous odor. The compound has a wide range of uses against various phytopathogenic fungi and has a quasi-systemic translocation behavior, a relatively short residence time within the plant and high photochemical stability. It can be used protective as well as curative and eradicative. BASF applied for approval of the product in Europe in 1995 .
In Germany, Austria and Switzerland, plant protection products with this active ingredient are approved.
safety instructions
Kresoxim-methyl is suspected of having a carcinogenic effect. It is very toxic to aquatic organisms and can have long-term harmful effects in the aquatic environment.
Web links
- Joint Meeting on Pesticide Residues (JMPR), Monograph for Kresoxim-methyl
- National Registration Authority for Agricultural and Veterinary Chemicals Australia: Evaluation of the new active KRESOXIM-METHYL in the product STROBY WG FUNGICIDE (PDF; 174 kB), June 2000
Individual evidence
- ↑ a b c Source: Expression of single chain antibodies against the fungicide kresoxim-methyl in transgenic tobacco plants (PDF; 4.2 MB), Michael Leps dissertation, July 2002.
- ↑ a b c d e data sheet Kresoxim-methyl, PESTANAL at Sigma-Aldrich , accessed on May 18, 2017 ( PDF ).
- ↑ a b c European Commission - Directorate General for Agriculture: Review report for the active substance kresoxim-methyl (PDF; 493 kB), October 16, 1998.
- ↑ a b EPA: Kresoxim-methyl .
- ↑ a b Entry for CAS no. 143390-89-0 in the GESTIS substance database of the IFA , accessed on January 20, 2012(JavaScript required) .
- ↑ Entry on kresoxim-methyl (ISO) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Wolfgang Krämer, Ulrich Schirmer, Peter Jeschke, Matthias Witschel: Modern Crop Protection Compounds . Wiley-VCH, 2011, ISBN 978-3-527-32965-6 ( page 616 in the Google book search).
- ↑ a b Stähler: MSDS Stroby WG (PDF; 124 kB).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on kresoxim-methyl in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 18, 2016.