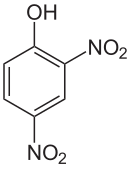
2,4-dinitrophenol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,4-dinitrophenol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 4 N 2 O 5 | ||||||||||||||||||
| Brief description |
yellow crystalline powder with a phenolic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 184.11 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.683 g cm −3 |
||||||||||||||||||
| Melting point |
108-112 ° C |
||||||||||||||||||
| pK s value |
4.09 |
||||||||||||||||||
| solubility |
heavy in water (6 g l −1 ) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
2,4-Dinitrophenol ( DNP for short ) is a yellow crystalline solid with a phenol-like odor. The structure consists of a benzene ring with a hydroxyl group (-OH) and two nitro groups (-NO 2 ) as substituents . It belongs to the group of dinitrophenols , a group of six constitutional isomers . It was first used in 1919. At that time it was used in France to make ammunition. 40% DNP and 60% TNT made an explosive mixture for artillery shells. Before DNP's toxicity was recognized, it found widespread use as an anti- obesity agent in the 1930s .
presentation
2,4-dinitrophenol formed from o - and p -nitrophenol by renewed nitration. It is an intermediate product on the way to picric acid .
2,4-Dinitrophenol can be formed in the atmosphere by the reaction of nitrate radicals with phenol or other aromatics.
properties
Physical Properties
2,4-Dinitrophenol is a yellow crystalline powder with a phenol-like odor. It crystallizes in the orthorhombic crystal system in the space group P 2 1 2 1 2 1 (space group no. 19) with the lattice parameters a = 610.6 pm , b = 2324 pm and c = 516.8 pm. In the unit cell contains four formula units .
Chemical properties
The weakly acidic character of the phenol is due to the mesomeric stabilization of the phenolate ion. The two nitro groups are electron-withdrawing due to the −M effect ; the phenolic OH bond is increasingly polarized. It therefore has a higher acidity compared to phenol. The pK s value of 4.09 is therefore correspondingly lower (phenol: 9.99).
In the atmosphere, 2,4-dinitrophenol reacts rapidly with photo-chemically generated hydroxyl radicals. It is also fickle during the night as it is oxidized by nitrate radicals. 2,4-Dinitrophenol is probably broken down in water under aerobic conditions; without oxygen, the breakdown begins at the nitro group.
Reactions
The bromination of 2,4-dinitrophenol with elemental bromine in glacial acetic acid leads to 2-bromo-4,6-dinitrophenol, which has a melting point of 118–119 ° C. Direct iodination with elemental iodine and mercury (II) oxide produces 2-iodo-4,6-dinitrophenol . Chlorination with sodium hypochlorite gives 2-chloro-4,6-dinitrophenol .
2,4-Dinitrophenol can be partially reduced to 2-amino-4-nitrophenol with sodium sulfide or ammonium sulfide . The complete reduction to 2,4-diaminophenol can, for. B. be carried out with tin , zinc or iron and hydrochloric acid or with elemental hydrogen on a nickel catalyst.
Biological effects
Mechanism of action
2,4-Dinitrophenol (DNP) is a proton ionophore (proton transporter) that acts as a decoupler for oxidative phosphorylation in the mitochondria of the cell .
This is where the utilization of carbohydrates and fatty acids ends in the so-called respiratory chain , which creates a chemiosmotic potential between the intermembrane space and the mitochondrial matrix and thus u. a. builds up a proton gradient between these two spaces, which is normally used to generate energy in the form of ATP .
“Decouplers” of oxidative phosphorylation such as DNP, on the other hand, break down this gradient again by absorbing protons from the intermembrane space, diffusing with them through the inner mitochondrial membrane into the matrix and releasing the protons there again.
In the case of the DNP, it then migrates back into the intermembrane space, stabilized by mesomerism, where the cycle begins again, but the energy that was stored in the proton gradient is lost as heat. In order to maintain or rebuild the gradient for operating the ATP synthesis , the cell must consequently increase its metabolic rate and, for example, metabolize more fatty acids and / or carbohydrates , so that the physiological effect of DNP is ultimately based on nothing more than an intensification of the fat. and carbohydrate metabolism.
Of particular physiological importance, the uncoupling of oxidative phosphorylation described is also in brown adipose tissue of newborns where they - though there by Thermogenin , an endogenous ion channel - protein also one - the inner mitochondrial membrane jitter-free heat production allows.
Toxic Effects
Unwanted effects of DNP manifest themselves in a drop in blood pressure, palpitations ( tachycardia ), cardiac arrhythmias , sudden cardiac death, shortness of breath ( dyspnea ), aspiration pneumonia , pulmonary edema, headache, restlessness, brain edema, coma, overheating , metabolic acidosis , transverse metabolic acidosis , muscular acidosis ( hyperthermia ) ( Rhabdomyolysis ), thyroid dysfunction , increased blood sugar level, abdominal pain, nausea, vomiting, cyanosis , increased breakdown of red blood cells ( haemolytic anemia ), changes in the blood pigment with disruption of oxygen transport ( methaemoglobinaemia ), destruction of the white blood cells ( agranulocytosis) → costmann's syndrome ( agranulocytosis ) Yellowish skin coloration, burning of the skin, cataracts , kidney failure , kidney failure, liver disorders, liver failure and ultimately multiple organ failure in the advanced stages of DNP poisoning.
Whether 2,4-dinitrophenol is carcinogenic (carcinogenic) remains unclear. In a previous study in mice, DNP showed no effect on the growth of induced skin cancer. A possible mutagenic property of 2,4-dinitrophenol was investigated in bacteria using the Ames test . It turns out that DNP had no mutagenic effect. A teratogenic effect of a DNP-like compound, dinoseb , has been shown in animal experiments.
In current medical studies, the lethal dose of 2,4-dinitrophenol is given as 1 to 3 grams. However, this is for a single dose and the pharmacokinetics of DNP are not precisely known. However, the half-life appears to be long enough for accumulation to take place. One study describes e.g. For example, a case in which a bodybuilder took DNP at 600 mg / day for 4 days and then died one day later. Most DNP deaths can probably be traced back to the fact that the cumulative effect was not taken into account. As always, people react differently, and if the personal lethal dose is 1 gram, then this amount in the body can be reached after a few days, even if one ingests significantly less per day. The most recent documented death in Germany is a 19-year-old schoolgirl who died in August 2006 after taking DNP once in the Agnes Karl Hospital in Laatzen.
So far no effective antidote has been discovered; in the case of DNP poisoning, only the symptoms can be treated supportively. However, there is known a case where a patient with DNP-induced hyperthermia could be successfully treated by repeated administration of dantrolene . Dantrolene is used as a remedy for malignant hyperthermia . Possibly the rapid administration could save the patient's life.
use
Illegal health related use
From observations of French workers in explosives factories during the First World War who came into contact with 2,4-dinitrophenol at work and who lost body weight in addition to considerable symptoms (dizziness, sweating and headaches), the hypothesis arose that obesity was caused by ingestion of 2,4-Dinitrophenol is to be treated. This was made particularly well known by the first study results under the pharmacologist Maurice L. Tainter. After brief initial problems, 2,4-dinitrophenol advanced to become a drug against obesity in the USA in the 1930s . The reason for this popularity was that daily doses of 3 to 5 mg 2,4-dinitrophenol per kg body weight increase the basal metabolic rate in a healthy person by up to 50%. In contrast to thyroid hormones, dinitro compounds do not attack the protein albumin contained in the cell tissue and fat is not broken down at the expense of the muscles. In addition, it does not increase blood pressure or pulse.
The use of DNP as a weight loss drug was increasingly questioned in the mid-1930s. For example, two doctors concluded from the data of one study that the therapeutic levels of DNP contributed very little to weight loss. In addition, more and more serious side effects (see section below) have been observed during therapy with DNP. Tainter, however, took the view that these were at most the rare consequences of hypersensitivity to DNP.
Because of its dangerousness and its simultaneously narrow therapeutic range , the American drug approval authority (FDA) finally withdrew the approval for the sale of drugs containing DNP in 1938 .
In the 1980s, 2,4-dinitrophenol was illegally reintroduced as an anti-obesity and anti-cancer drug. Products containing DNP were sold in the form of capsules. The death of a wrestler from taking these pills prompted an investigation which resulted in a court hearing. In the course of the ruling, DNP was withdrawn from the market as a weight loss drug. To date, there has been no approval of a DNP-containing dietary supplement or drug by the American drug approval authority. Nonetheless, DNP is still used by bodybuilders and athletes today to help lose body fat quickly.
In Germany, the distribution of DNP as a diet product is prohibited by law. In August 2006, a 19-year-old woman died there after taking about one gram of this substance. She was hospitalized with acute heart problems, hot flashes and shortness of breath, where she died a day later.
Technical use
2,4-Dinitrophenol is used in the synthesis of dyes and wood preservatives , photo chemicals , insecticides , explosives, pH indicators, as well as to represent picric acid and amidol .
Legal
The compound is dry or with less than 15% water mass fraction an explosive within the meaning of Article 1 Paragraphs 2 and 3 of Directive 93/15 / EEC and the Explosives Act .
literature
- E. Colman: Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. In: Regul. Toxicol. Pharmacol. , 2007, 48 (2), pp. 115-117; PMID 17475379 ; doi : 10.1016 / j.yrtph.2007.03.006 .
- J. Bell: Etude biologique des produits dinitres chez l'homme. In: Medecine , 1939, 19 , pp. 749-754; PDF (free full-text access, English translation Biological Study of Dinitro Drugs in Humans by Robert Ames 1996).
Web links
- General information
- Nitrophenolic and Nitrocresolic Herbicides (Chapter 11); PDF .
Individual evidence
- ↑ a b c d e f g h Entry on 2,4-dinitrophenol in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Entry on 2,4-dinitrophenol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ^ A b c Environmental Protection Agency: Federal Register , Vol. 56 (44) (March 1991), p. 9564.
- ↑ T. Kagawa, R. Kawai, S. Kashino, M. Haisa: The crystal and molecular structure of 2,4-dinitrophenol. In: Acta Cryst. , 1976, B32 , pp. 3171-3175; doi : 10.1107 / S0567740876009886 .
- ↑ K. Dudova, F. Castek, V. Machacek, P. Simunek: Preparation of Substituted Methyl o-Nitrophenyl Sulfides. In: Molecules , 2002, 7 , pp. 7-17; doi : 10.1001 / jama.1935.02760270015006 .
- ^ HH Hodgson: The Iodination of o-nitrophenol. In: J. Chem. Soc. , 1927, pp. 1141-1144; doi : 10.1039 / JR9270001141 .
- ^ HE Armstrong: Observations on the nitrochlorophenols. In: J. Chem. Soc. , 1872, 25 , pp. 12-17; doi : 10.1039 / JS8722500012 .
- ^ WW Hartman, HL Silloway: 2-Amino-4-Nitrophenol In: Organic Syntheses . 25, 1945, p. 5, doi : 10.15227 / orgsyn.025.0005 ; Coll. Vol. 3, 1955, p. 82 ( PDF ).
- ↑ K. Auwers, H. Röhrig: About some new oxyazo bodies and triphendioxazine derivatives. In: Reports of the German Chemical Society , 1897, 30 , pp. 988–998; Full text
- ^ WE Bradt: The Catalytic Preparation of 2-4 Diaminophenol. In: J. Phys. Chem. , 1930, 34 (12), pp. 2711-2718; doi : 10.1021 / j150318a006 .
- ^ F. Stenbäck and H. Garcia: Studies on the modifying effect of dimethyl sulfoxide and other chemicals on experimental skin tumor induction. In: Ann. NY Acad. Sci. , 1975, 243 , pp. 209-227; PMID 1055541 ; doi : 10.1111 / j.1749-6632.1975.tb25359.x .
- ↑ D. de Flora: Study of 106 organic and inorganic compounds in the Salmonella / microsome test. In: Carcinogenesis , 1981, 2 (4), pp. 283-298; PMID 7023727 ; doi : 10.1093 / carcin / 2.4.283 .
- ^ JE Gibson: Teratology studies in mice with 2-sec-butyl-4,6-dinitrophenol (dinoseb). In: Food and Cosmetics Toxicology , 1973, 11 (1), pp. 31-43; PMID 4716128 ; doi : 10.1016 / 0015-6264 (73) 90059-X .
- ↑ JC Suozzi, CM Rancont, RB McFee: DNP 2,4-dinitrophenol: a deadly way to lose weight. In: Journal of Emergency Medical Services , 2005, 30 , pp. 82-89; PMID 15662347 .
- ^ RB McFee, TR Caraccio, MA McGuigan, SA Reynolds, P. Bellanger: Dying to be thin: a dinitrophenol related fatality. In: Vet. Hum. Toxicol. , 2004, 46 , pp. 251-254; PMID 15487646 .
- ↑ a b 19-year-old dies from an overdose of diet powder. In: Welt online , September 18, 2007.
- ^ S. Kumar, K. Barker, D. Seger: Dinitrophenol-Induced Hyperthermia Resolving With Dantrolene Administration. In: Abstracts of the North American Congress of Clinical Toxicology. In: J Toxicol Clin Toxicol , 2002, 40 , p. 689; PDF .
- ↑ C. Sieg Mueller, R. Narasimhaiah: Fatal 2,4-dinitrophenol poisoning ... coming to a hospital near you. In: Emerg. Med. J. , 2010, 27 (8), pp. 639-640; PMID 20511642 ; doi : 10.1136 / emj.2009.072892 .
- ^ E. Colman: Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. In: Regul. Toxicol. Pharmacol. , 2007, 48 (2), pp. 115-117; PMID 17475379 ; doi : 10.1016 / j.yrtph.2007.03.006 .
- ^ WC Cutting, ML Tainter: Actions of Dinitrophenol. In: Proc. Soc. Exper. Biol. Med. , 1932, 29 , pp. 1268-1269.
- ↑ JM Strang and FA Evans: An evaluation of dinitrophenol as an aid in weight reduction. In: Journal of the American Medical Association , 1936, 1 , pp. 1957-1963; Abstract .
- ↑ CM MacBryde and BL Taussig: Functional changes in liver, heart, and muscle, and loss of dextrose tolerance resutling from dinitrophenol. In: Journal of the American Medical Association , 1935, 6 , pp. 13-17; Abstract .
- ↑ J. Bartlett, M. Brunner, K. Gough: Deliberate poisoning with dinitrophenol (DNP): an unlicensed weight loss pill. In: Emerg. Med. J. , 2010, 27 (2), pp. 159-160; PMID 20156878 ; doi : 10.1136 / emj.2008.069401 .
- ↑ K. Wagner: 1-2-4 dinitrophenol poisoning : In: Fühner-Wieland's collection of poisoning cases , 1936, 7 , pp. C9 – C20; doi : 10.1007 / BF02453003 .
- ↑ Wilfried Dubbels: Dangerous Slimming Makers. In: Pharmaceutical newspaper online, issue 44, 2007.
- ↑ A. Tewari, T. Ali, J. O'Donnell, MS Butt: Weight loss and 2,4-dinitrophenol poisoning. In: Br. J. Anaesth. , 2009, 102 (4), pp. 566-567; PMID 19286775 ; PDF .
- ↑ Law on Explosive Substances (Sprengstoffgesetz - SprengG), Annex III List of Explosives in accordance with Section 3 Paragraph 1 No. 1 PDF






