Succinate dehydrogenase
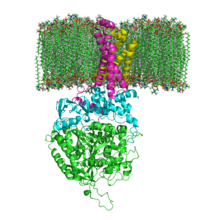
The enzyme succinate dehydrogenase (SDH), more precisely succinate: ubiquinone oxidoreductase (systematic name), also called complex II , is an enzyme complex consisting of four subunits. It is the only membrane-bound protein of the citric acid cycle and, as complex II of the respiratory chain, is directly integrated into the electron transport chain of the inner mitochondrial membrane. The enzyme catalyzes
the oxidation of succinate to fumarate with FAD as oxidant :
the simultaneous transport of two electrons across the membrane boundary :
- 2e - (inside) + FAD H 2 2e - (outside) + FAD + 2H +
with the help of these electrons the reduction of ubiquinone ( coenzyme Q ) to ubiquinol ( EC 1.3.5.1 ):
- Q + 2H + + 2e - Q H 2
Mutations in one of the four coding genes can be responsible for hereditary metabolic diseases in humans (see table).
structure
In humans, the protein consists of four protein subunits (see table).
| gene | protein | Amino acids | UniProt | OMIM | comment |
|---|---|---|---|---|---|
| SDHA | Flavoprotein subunit | 621 | P31040 | 600857 | Complex II deficiency |
| SDHB | Iron-sulfur protein subunit | 252 | P21912 | 185470 | Pheochromocytoma , paraganglioma , Carney-Stratakis syndrome |
| SDHC | Cytochrome b560 subunit | 140 | Q99643 | 602413 | 4 isoforms; Paraganglioma , Carney Stratakis Syndrome |
| SDHD | Cytochrome b560 subunit | 103 | O14521 | 602960 | Pheochromocytoma , paraganglioma , Carney-Stratakis syndrome , Leigh syndrome, and colorectal cancer |
In evolutionary terms , the flavoprotein is the oldest subunit and homologous proteins can therefore already be found in many bacteria ( EC 1.3.99.1 ). With the evolution of the eukaryotes, the iron-sulfur protein was added, and with the vertebrates and the then added cytochrome, the complex was anchored in the membrane.
function
The enzyme catalyzes the oxidation of succinate to fumarate and the reduction of ubiquinone ( coenzyme Q ) to ubiquinol. In contrast to NADH , the FADH 2 that is produced as a hydrogen carrier during this oxidation in the citric acid cycle does not occur freely, but is bound as a prosthetic group to the SdhA subunit of the enzyme complex. Its reoxidation takes place through a chain of one-electron transfers starting with the three iron-sulfur clusters Fe 2 S 2 , Fe 4 S 4 , Fe 3 S 4 of the SdhB subunit, up to cytochrome b 560 (in mitochondria) or cytochrome b 556 ( in bacteria) of the subunits SdhC and SdhD. This ultimately reduces the quinoid - i.e. oxidized - form of coenzyme Q (ubiquinone) into the phenolic form, ubiquinol.
Since the iron complexes involved are only capable of one-electron transfers , the reoxidation of FADH 2 to FAD or the reduction of ubiquinone, which each provide or require two electrons, takes place in two stages via radical , but stable, semichinoid intermediate stages . The binding pocket for succinate is in the subunit SdhA; that for ubiquinone is formed by the three subunits SdhB, SdhC and SdhD. Due to its lipophilic polyisoprenyl chain , ubiquinone / ubiquinol is membrane- soluble. It therefore serves as a mobile electron carrier from complex II to complex III of the respiratory chain.
The succinate dehydrogenase redox chain :
The balance of this step of the citric acid cycle results in a transfer of two electrons from succinate to ubiquinone. However, this enzyme does not transport protons.
Inhibitor
Individual evidence
- ↑ UniProt P31040
- ↑ UniProt P21912
- ↑ UniProt Q99643
- ↑ UniProt O14521
- ↑ Fumarate reductase / succinate dehydrogenase FAD-binding site
See also
Web links
- Video: succinate dehydrogenase . Institute for Scientific Film (IWF) 2005, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / C-12757 .