Glyceraldehyde-3-phosphate dehydrogenase
| Glyceraldehyde-3-phosphate dehydrogenase | ||
|---|---|---|

|
||
| Surface / ribbon model of the GAPDH tetramer, according to PDB 1U8F | ||
| Properties of human protein | ||
| Mass / length primary structure | 334 aa; 35.9 kDa | |
| Secondary to quaternary structure | Homotetramer | |
| Cofactor | PRKCI, sulfate | |
| Identifier | ||
| Gene names | GAPDH ; GAPD | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.2.1.12 , oxidoreductase | |
| Response type | Phosphorylation | |
| Substrate | D -Glyceraldehyde-3-phosphate + phosphate + NAD (+) | |
| Products | 3- phospho - D -glyceroyl phosphate + NADH | |
| Occurrence | ||
| Homology family | CLU_030140_0_3 | |
| Parent taxon | Creature | |
| Orthologue | ||
| human | chicken | |
| Entrez | 2597 | 374193 |
| Ensemble | ENSG00000111640 | ENSGALG00000014442 |
| UniProt | P04406 | P00356 |
| Refseq (mRNA) | NM_002046 | NM_204305 |
| Refseq (protein) | NP_002037 | NP_989636 |
| Gene locus | Chr 12: 6.51 - 6.52 Mb | Chr 1: 80.09 - 80.09 Mb |
| PubMed search | 2597 |
374193
|
The glyceraldehyde-3-phosphate dehydrogenase ( GAPDH ) is an enzyme of glycolysis , and therefore indispensable for all living beings. It catalyzes the conversion of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate . During this reaction, an energy-rich phosphate bond is built up, which is transferred to ADP in the subsequent glycolysis step, creating ATP. In addition, an NAD + is converted to NADH / H + in the catalyzed reaction .
structure
Under normal cellular conditions, the cytoplasmic GAPDH exists primarily as a tetramer . This form consists of four identical 37 kDa subunits, each of which contains a single catalytic thiol group and is crucial for the catalytic function of the enzyme. The GAPDH in the cell nucleus has an elevated isoelectric point (pI) at pH 8.3 to 8.7. The cysteine residue Cys152 in the active center of the enzyme is necessary for the induction of apoptosis by oxidative stress . In particular, post-translational modifications of the cytoplasmic GAPDH contribute to its functions outside of glycolysis.
Catalytic Mechanism
The following enzymological experiments have contributed to understanding the reaction mechanism of GAPDH :
- GAPDH was rendered ineffective by alkylation with stoichiometric amounts of iodoacetate . The presence of carboxymethylcysteine in the hydrolyzate of the resulting alkylated enzyme shows that GAPDH has a cysteine residue in the active site, the thiol group of which plays a role in the mechanism.
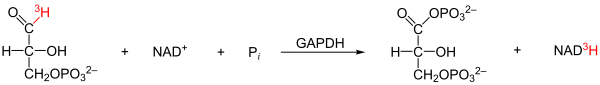
- GAPDH quantitatively transfers a tritium from the C1 atom of GAP to NAD + . This proved that the reaction proceeds via hydride transfer.
- GAPDH catalyzes the exchange of 32 P between P i and acetyl phosphate. Such isotope exchange reactions indicate an acyl-enzyme as an intermediate product , ie that the acyl group forms a covalent complex with the enzyme, similar to the acyl-enzyme intermediate in the reaction mechanism of serine proteases .
The main problem of the reaction is the splitting off of the H - ion ( hydride ion ) from the aldehyde group of the glyceraldehyde-3-phosphate. This is energetically unfavorable since the carbon in the aldehyde group has a partial positive charge. Through a sulfhydryl group of a cysteine of the enzyme is via a covalent bond , a nucleophilic residue introduced. The nucleophile attacks the carbonyl carbon of glyceraldehyde-3-phosphate (GAP) to form a thio-hemiacetal. After abstraction of the proton of the hydroxyl group on the C1 atom of GAP by a base, the hydride ion dissolves and a thioester is formed between the enzyme and the substrate (oxidation). This energy-rich compound is used in the subsequent reaction to bind an inorganic phosphate and to convert the intermediate product into 1,3-bisphosphoglycerate (phosphorylation). The hydride ion now binds to an NAD + that is non-covalently bound to a Rossmann fold , so that NADH / H + is formed. This loosens from the bond with the enzyme and is replaced by an NAD + because the positive charge of the NAD + polarizes the thioester intermediate in order to facilitate the attack by the orthophosphate. The catalyzed reaction is energetically very important. The resulting mixed anhydride of phosphoric and carboxylic acids is used in the subsequent reaction to form ATP . From the NADH / H + , in turn, ATP can be formed in the respiratory chain . Because cells only have a limited amount of NAD + , glycolysis would stop if the NADH produced in glycolysis were not continuously reoxidized. Therefore, NAD + is regenerated from the anaerobic breakdown of pyruvate.

The reaction catalyzed by glyceraldehyde-3-phosphate dehydrogenase is actually the sum of two processes: the oxidation of glyceraldehyde-3-phosphate to 3-phosphoglycerate by NAD + and the phosphorylation of 3-phosphoglycerate to 1,3-bisphosphoglycerate ( dehydration ).
The first reaction is thermodynamically quite favorable with a change in standard free enthalpy ( ) of about -50 kJ / mol. The second reaction is very unfavorable with a standard free enthalpy of about +50 kJ / mol. A high activation energy would therefore be required for the second reaction, which is why it would not proceed at a biologically significant rate.
Therefore, the two reactions must be coupled so that the overall reaction can take place. The coupling takes place via a thioester intermediate which is bound to the enzyme by a thioester bond. Thioesters are high-energy compounds found in many biochemical metabolic pathways. The free energy of the thioester intermediate is greater than that of the free carboxylic acid. This means that most of the free enthalpy that was released during the oxidation reaction is retained.
Features and function
All glycolysis steps take place in the cytosol, as does the reaction catalyzed by GAPDH. In red blood cells , GAPDH and several other glycolytic enzymes form certain enzyme complexes on the inside of the cell membrane . The process seems to be regulated by phosphorylation and oxygenation. The approach of several glycolytic enzymes is expected to greatly increase the overall rate of glucose breakdown. Recent studies have also shown that GAPDH is iron-dependently expressed on the outside of the cell membrane , where it plays a role in maintaining cellular iron homeostasis , particularly as a chaperone protein for labile heme in cells.
Transcription and apoptosis
GAPDH can activate transcription itself . The OCA-S transcription coactivator complex contains GAPDH and lactate dehydrogenase , two proteins that were previously thought to be only involved in metabolism. GAPDH moves between the cytosol and the cell nucleus and can thus combine the metabolic state with gene transcription.
In 2005, Hara et al. demonstrated that GAPDH initiates apoptosis. Initiation is mediated through GAPDH binding to DNA . The study showed that GAPDH is S -nitrosylated by nitric oxide in response to cell stress , causing it to bind to the protein SIAH1, a ubiquitin ligase . The complex moves into the nucleus where SIAH1 targets core proteins for breakdown, initiating a controlled shutdown of the cells. In a subsequent study, the group showed that selegiline , which was used clinically to treat Parkinson's disease , greatly reduced the apoptotic effects of GAPDH by preventing its S -nitrosylation and could therefore be used as a drug.
Metabolism switch
GAPDH acts as a reversible metabolic switch under oxidative stress. When cells are exposed to certain oxidizing agents , they need excessive amounts of the antioxidant cofactor NADPH . In the cytosol, NADPH is produced from the reduction of NADP + by several enzymes , three of which catalyze the first steps of the pentose phosphate pathway . Oxidant treatments cause inactivation of GAPDH. This inactivation redirects the metabolic flow from glycolysis to the pentose phosphate pathway so that the cell can produce more NADPH. Under stressful conditions, NADPH is required by some antioxidant systems, including glutaredoxin and thioredoxin , and is essential for the recycling of glutathione .
inhibition
The formation of the energy-rich anhydride can be inhibited by arsenic . AsO 4 3− binds to the GAPDH analogously to phosphate . NADH continues to be formed. However, the bond between the carboxylate resulting from the oxidation of the aldehyde and the arsenate is very unstable, so that the mixed anhydride breaks down to 3-phosphoglycerate . As a result, an energy-fixing step in glycolysis is skipped, which contributes to the poisonous effect of arsenic.
More functions
GAPDH also appears to be involved in vesicle transport from the endoplasmic reticulum (ER) to the Golgi apparatus , which is part of the transport route for secreted proteins. GAPDH has been found to be recruited by Rab2 into the ER's vesicular-tubular clusters, where it contributes to the formation of COPI vesicles . GAPDH is activated by tyrosine phosphorylation by tyrosine kinase Src .
Since the GAPDH gene is stably and constitutively expressed to a high degree in most tissues and cells, it is considered to be a household gene . For this reason, GAPDH is widely used by biological researchers as a load control for Western blotting and as a control for qPCR . However, researchers reported different GAPDH regulations under certain conditions. For example, the transcription factor MZF-1 has been shown to regulate the GAPDH gene. Therefore, the use of GAPDH as a cargo control must be carefully considered.
Clinical significance
cancer
GAPDH is overexpressed in several human cancers, such as skin melanoma , and its expression has a positive correlation with tumor progression. Its glycolytic and anti-apoptotic functions contribute to the proliferation and protection of tumor cells and promote carcinogenesis . In particular, GAPDH protects against telomere shortening induced by chemotherapeutic drugs that stimulate the sphingolipid ceramide . Conditions such as oxidative stress also impair GAPDH function and lead to cell aging and death. In addition, the removal of GAPDH induces senescence in tumor cells, which represents a novel therapeutic strategy for controlling tumor growth.
Neurodegenerative Disease
GAPDH is implicated in several neurodegenerative diseases and disorders, primarily through interactions with other proteins specific to that disease or disorder. These interactions can affect not only the energy metabolism but also other GAPDH functions. For example, GAPDH interactions with the β- amyloid precursor protein (BetaAPP) could impair the function of the cytoskeleton or membrane transport , while interactions with huntingtin could impair the function of apoptosis, nuclear tRNA transport, DNA replication and DNA repair could affect. In addition, GAPDH nuclear translocation (transport through the nuclear pores of the cell nucleus) has been reported in Parkinson's disease (PD), and several anti-apoptotic PD drugs, such as rasagiline, have been reported to work by preventing GAPDH nuclear translocation. It is believed that hypometabolism (decreased metabolic rate) contributes to Parkinson's disease. However, the exact mechanisms underlying GAPDH's involvement in neurodegenerative diseases have yet to be clarified. The SNP rs3741916 in the 5'-UTR of the GAPDH gene can be associated with late-onset Alzheimer's disease .
Genes, proteins and metabolites are linked to the respective articles. The metabolic pathway can be edited at WikiPathways :
Web links
Individual evidence
- ↑ a b c C. Tristan, N. Shahani, TW Sedlak, A. Sawa: The diverse functions of GAPDH: views from different subcellular compartments. In: Cellular signaling. Volume 23, number 2, February 2011, pp. 317-323, doi: 10.1016 / j.cellsig.2010.08.003 , PMID 20727968 , PMC 3084531 (free full text) (review).
- ↑ a b c d C. Nicholls, H. Li, JP Liu: GAPDH: a common enzyme with uncommon functions. In: Clinical and experimental pharmacology & physiology. Volume 39, Number 8, August 2012, pp. 674-679, doi: 10.1111 / j.1440-1681.2011.05599.x , PMID 21895736 (review).
- ^ Donald Voet, Judith G. Voet, Charlotte W. Pratt: Fundamentals of Biochemistry . Life at the Molecular Level. 5th edition. John Wiley & Sons , Hoboken, NJ 2016, ISBN 978-1-118-91840-1 , pp. 489 ( limited preview in Google Book search).
- ^ A b David L. Nelson, Albert L. Lehninger, Michael M. Cox: Lehninger Principles of Biochemistry . 5th edition. WH Freeman, 2008, ISBN 978-1-4292-0892-5 , pp. 530 .
- ^ Stryer, Lubert: Biochemie , Spectrum of Science Verlag, 4th edition, Heidelberg 1996, p. 526.
- ↑ a b Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto jr .: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 465–466 ( limited preview in Google Book Search).
- ↑ Florian Horn et al .: Human Biochemistry , Stuttgart 2003, ISBN 3-13-130883-4 , p. 80.
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto, Jr.: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 470 ( limited preview in Google Book search).
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto, Jr.: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 465 ( limited preview in Google Book search).
- ↑ E. DCE L. Olivier-Deyris, E. Fanchon, C. Corbier, G. Branlant, O. Dideberg: Comparison of the structures of wild-type and a N313T mutant of Escherichia coli glyceraldehyde 3-phosphate dehydrogenases: implication for NAD binding and cooperativity. In: Journal of molecular biology. Volume 257, Number 4, April 1996, pp. 814-838, doi : 10.1006 / jmbi.1996.0204 , PMID 8636984 .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto, Jr.: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 464–465 ( limited preview in Google Book search).
- ↑ ME Campanella, H. Chu, PS Low: Assembly and regulation of a glycolytic enzyme complex on the human erythrocyte membrane. In: Proceedings of the National Academy of Sciences . Volume 102, number 7, February 2005, pp. 2402-2407, doi: 10.1073 / pnas.0409741102 , PMID 15701694 , PMC 549020 (free full text).
- ↑ MA Sirover: Structural analysis of glyceraldehyde-3-phosphate dehydrogenase functional diversity. In: The international journal of biochemistry & cell biology. Volume 57, December 2014, pp. 20-26, doi: 10.1016 / j.biocel.2014.09.026 , PMID 25286305 , PMC 4268148 (free full text) (review).
- ↑ S. Kumar, N. Sheokand, MA Mhadeshwar, CI Raje, Raje M.: Characterization of glyceraldehyde-3-phosphate dehydrogenase as a novel transferrin receptor. In: The international journal of biochemistry & cell biology. Volume 44, Number 1, January 2012, pp. 189-199, doi: 10.1016 / j.biocel.2011.10.016 , PMID 22062951 .
- ↑ EA Sweeny, AB Singh, R. Chakravarti, O. Martinez-Guzman, A. Saini, MM Haque, G. Garee, PD Dans, L. Hannibal, AR Reddi, DJ Stuehr: Glyceraldehyde -3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. In: Journal of Biological Chemistry . Volume 293, number 37, 09 2018, pp. 14557-14568, doi: 10.1074 / jbc.RA118.004169 , PMID 30012884 , PMC 6139559 (free full text).
- ↑ L. Zheng, RG Roeder, Y. Luo: S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. In: Cell . Volume 114, Number 2, July 2003, pp. 255-266, doi: 10.1016 / s0092-8674 (03) 00552-x , PMID 12887926 .
- ↑ MR Hara, N. Agrawal, SF Kim, MB Cascio, M. Fujimuro, Y. Ozeki, M. Takahashi, JH Cheah, SK Tankou, LD Hester, CD Ferris, SD Hayward, SH Snyder, A. Sawa: S- nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. In: Nature cell biology. Volume 7, Number 7, July 2005, pp. 665-674, doi: 10.1038 / ncb1268 , PMID 15951807 .
- ↑ MR Hara, B. Thomas, MB Cascio, BI Bae, LD Hester, VL Dawson, TM Dawson, A. Sawa, SH Snyder: Neuroprotection by pharmacologic blockade of the GAPDH death cascade. In: Proceedings of the National Academy of Sciences . Volume 103, number 10, March 2006, pp. 3887-3889, doi: 10.1073 / pnas.0511321103 , PMID 16505364 , PMC 1450161 (free full text).
- ↑ AR Agarwal, L. Zhao, H. Sancheti, IK Sundar, I. Rahman, E. Cadenas: Short-term cigarette smoke exposure induces reversible changes in energy metabolism and cellular redox status independent of inflammatory responses in mouse lungs. In: American Journal of Physiology - Lung Cellular and Molecular Physiology. Volume 303, number 10, November 2012, pp. L889-L898, doi: 10.1152 / ajplung.00219.2012 , PMID 23064950 .
- ↑ M. Ralser, MM Wamelink, A. Kowald, B. Gerisch, G. Heeren, EA Struys, E. Klipp, C. Jakobs, M. Breitenbach, H. Lehrach, S. Krobitsch: Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. In: Journal of biology. Volume 6, number 4, December 2007, p. 10, doi: 10.1186 / jbiol61 , PMID 18154684 , PMC 2373902 (free full text).
- ↑ Bergmeyer, Hans Ulrich: Methods of enzymatic analysis Volume 1, Verlag Chemie, 3rd edition, Weinheim 1974, p. 115.
- ^ Stryer, Lubert: Biochemie , Spektrum der Wissenschaft Verlag, 4th edition, Heidelberg 1996, p. 527.
- ↑ EJ Tisdale, CR Artalejo: A GAPDH mutant defective in Src-dependent tyrosine phosphorylation impedes Rab2-mediated events. In: Traffic. Volume 8, number 6, June 2007, pp. 733-741, doi: 10.1111 / j.1600-0854.2007.00569.x , PMID 17488287 , PMC 3775588 (free full text).
- ↑ RD Barber, DW Harmer, RA Coleman, BJ Clark: GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. In: Physiological genomics. Volume 21, Number 3, May 2005, pp. 389-395, doi: 10.1152 / physiolgenomics.00025.2005 , PMID 15769908 .
- ↑ RT Piszczatowski, BJ Rafferty, A. Rozado, S. Tobak, NH Lents: The glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) is regulated by myeloid zinc finger 1 (MZF-1) and is induced by calcitriol. In: Biochemical and biophysical research communications. Volume 451, Number 1, August 2014, pp. 137-141, doi: 10.1016 / j.bbrc.2014.07.082 , PMID 25065746 .
- ↑ D. Ramos, A. Pellín-Carcelén, J. Agustí, A. Murgui, E. Jordá, A. Pellín, C. Monteagudo: Deregulation of glyceraldehyde-3-phosphate dehydrogenase expression during tumor progression of human cutaneous melanoma. In: Anticancer Research . Volume 35, Number 1, January 2015, pp. 439-444, PMID 25550585 .
- ↑ D. Wang, DR Moothart, DR Lowy, X. Qian: The expression of glyceraldehyde-3-phosphate dehydrogenase associated cell cycle (GACC) genes correlates with cancer stage and poor survival in patients with solid tumors. In: PLOS ONE . Volume 8, number 4, 2013, p. E61262, doi: 10.1371 / journal.pone.0061262 , PMID 23620736 , PMC 3631177 (free full text).
- ↑ M. Phadke, N. Krynetskaia, A. Mishra, E. Krynetskiy: Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. In: Biochemical and biophysical research communications. Volume 411, number 2, July 2011, pp. 409-415, doi: 10.1016 / j.bbrc.2011.06.165 , PMID 21749859 , PMC 3154080 (free full text).
- ↑ JL Mazzola, MA Sirover: Alteration of intracellular structure and function of glyceraldehyde-3-phosphate dehydrogenase: a common phenotype of neurodegenerative disorders? In: Neurotoxicology. Volume 23, Numbers 4-5, October 2002, pp. 603-609, PMID 12428732 (Review).
- ^ M. Allen, C. Cox, O. Belbin, L. Ma, GD Bisceglio, SL Wilcox, CC Howell, TA Hunter, O. Culley, LP Walker, MM Carrasquillo, DW Dickson, RC Petersen, NR Graff-Radford, SG Younkin, N. Ertekin-Taner: Association and heterogeneity at the GAPDH locus in Alzheimer's disease. In: Neurobiology of aging. Volume 33, number 1, January 2012, pp. 203.e25-203.e33, doi: 10.1016 / j.neurobiolaging.2010.08.002 , PMID 20864222 , PMC 3017231 (free full text).