Rab proteins
The family of Rab proteins ("Ras-related in brain") belongs to the Ras superfamily of monomeric G proteins ( GTPases ) and is largely conserved in eukaryotes . There are over 60 known Rab proteins in humans, each of which has a specific subcellular localization and plays a role in intracellular vesicle sorting between the various compartments. In general, a distinction is made between an active form, in which GTP is bound, and an inactive form containing GDP .
properties
Rabs are peripheral membrane proteins that protrude from the vesicle surface into the cytosol and are fixed in the membrane via a prenyl anchor . After synthesis, a Rab protein is first bound by the Rab escort protein (REP) and presented to the enzyme geranylgeranyltransferase, which usually prenylates two C-terminal cysteine residues. In the following, REP functions as a kind of chaperone , which shields the hydrophobic part of the molecule from the cytosol and transports it to the membrane.
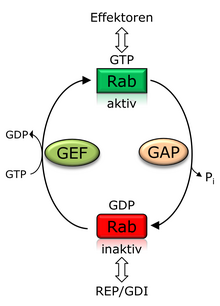
As usual for G proteins, Rab proteins go through a so-called GTPase cycle. Rab proteins are activated by exchanging GDP for GTP with the aid of a GTP exchange factor (GEF, guanine nucleotide exchange factor ). The GEF ensures the release of the bound GDP so that the GTP, which is present in a higher cytosolic concentration, can bind to the Rab protein. The binding of GTP causes conformational changes in the regions of the Rab protein called Switch I and Switch II , so that it can subsequently interact with effector proteins. The vesicle transport in the cell (between the organelles and between them and the plasma membrane) is regulated via the effector proteins by influencing vesicle constriction, movement and fusion. The effector proteins include enzymes , proteins of the cytoskeleton as well as other proteins that are involved in the directed membrane fusion. Inactivation takes place through GTP hydrolysis, which is supported by a so-called GTPase-activating protein (GAP). In the inactive state, the Rab protein can be extracted from the membrane by the so-called GDP dissociation inhibitor (GDI), which has a structural similarity to REP, and kept soluble in the cytosol. A cycle of localization between the cytosol and the membrane is coupled to the change between GTP / GDP binding. The activation of the Rab protein essentially takes place on the donor membrane, so that active Rab proteins are membrane-localized. After vesicular transport has taken place, the Rab proteins are inactivated on the acceptor membrane and transferred back into the cytosol by GDI so that they are available for further recruitment processes to the donor membrane. In the inactive GDP-bound state, Rab proteins are therefore predominantly cytosolic.
The more than 60 different human Rab proteins are specifically localized in the cell and regulate membrane transport between certain compartments. A selection of locations and functions is shown in the following table.
| protein | Organelle (localization) | corresponding membrane transport path / function |
|---|---|---|
| Rab1 | Endoplasmic reticulum (ER) and Golgi apparatus | Transport from the ER to the Golgi |
| Rab2 | cis -Golgi network | Transport from the ER to the Golgi |
| Rab3A | synaptic vesicles , secretory granules | Exocytosis, neurotransmitter release |
| Rab4 | early endosomes | Protein recycling, transport to the plasma membrane |
| Rab5A-C | Plasma membrane , clathrin- enveloped vesicles, early endosomes | Fusion of early endosomes |
| Rab6 | medial and trans -Golgi network (TGN) | Transport of endosomes to the Golgi apparatus, within the Golgi and from the Golgi to the ER |
| Rab7 | late endosomes , lysosomes , melanosomes , phagosomes | Transport from late endosomes to lysosomes |
| Rab8 | secretory vesicles, cell membrane, cilia | Exocytosis, transport from TGN and recycling endosomes to the plasma membrane |
| Rab9 | late endosomes, TGN | Transport later endosomes to the TGN |
| Rab27 | Melanosomes | Exocytosis |
The mechanism by which the various Rab proteins are localized to their specific donor membranes is not yet fully understood. But there are different models:
- An early model (Chavrier et al., 1991) implied that the hypervariable region of the Rab proteins in the C-terminal area may be responsible for the localization. This was shown by replacing the C-terminus of Rab5 with that of Rab7 and changing the location accordingly.
- In addition, a so-called GDI displacement factor (GDF) (Yip3) was identified. This is an integral membrane protein that is supposed to ensure the release of the Rab protein from the complex with GDI and the subsequent integration of the Rab protein into the target membrane.
- A third model sees the GEFs as a sufficient factor for the localization of the Rab proteins.
Diseases
A variant of Griscelli syndrome is caused by a point mutation in the gene that encodes Rab27a. The transport of melanosomes to the cell periphery and the secretion of lytic granules from cytotoxic T cells are disturbed. Rab25 is considered to be a promoter for tumor development.
Choroideremia is an X-linked inherited disease that can lead to retinal epithelium degeneration and blindness. The REP isoform REP-1 is affected, which is specifically responsible for the prenylation of Rab27a in the retina.
A mutation in GDI-α, an isoform mainly found in synapses of the CNS , can lead to X-linked inherited mental retardation, as the recycling of Rab is restricted.
See also
swell
- H. Stenmark, VM Olkkonen: The Rab GTPase family. In: Genome biology. Volume 2, number 5, 2001, S. REVIEWS3007, PMID 11387043 , PMC 138937 (free full text) (review).
- H. Stenmark: Rab GTPases as coordinators of vesicle traffic. In: Nat Rev Mol Cell Biol. 10 (8), 2009, pp. 513-525. PMID 19603039
- AH Hutagalung, PJ Novick: Role of Rab GTPases in Membrane Traffic and Cell Physiology. In: Physiol Rev. 91, 2011, pp. 119-149. PMID 21248164
Individual evidence
- ↑ P. Chavrier, JP Gorvel, E. Stelzer, K. Simons, J. Gruenberg, M. Zerial: Hypervariable C-terminal domain of rab proteins acts as a targeting signal. In: Nature. 1991, 353 (6346), pp. 769-772. PMID 1944536
- ↑ U. Sivars, D. Aivazian, SR Pfeffer: Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. In: Nature. 2003, 425 (6960), pp. 856-859. PMID 14574414
- ↑ YW Wu, LK Oesterlin, KT Tan, H. Waldmann, K. Alexandrov, RS Goody: Membrane targeting mechanism of Rab GTPases elucidated by semisynthetic protein probes. In: Nat Chem Biol. 2010, 6 (7), pp. 534-540. PMID 20512138 .
- ↑ D Kessler, GC Gruen, D Heider, J Morgner, H Reis, KW Schmid, V Jendrossek: The action of small GTPases Rab11 and Rab25 in vesicle trafficking during cell migration. . In: Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology . 29, No. 5-6, 2012, pp. 647-56. PMID 22613965 .