SNARE (protein)
| Parent |
| Cell membrane |
| Gene Ontology |
|---|
| QuickGO |
SNARE complexes ( Engl. Abbreviation for: s oluble N - ethylmaleimide -sensitive-factor a ttachment re ceptor) are protein complexes in vesicles of eukaryotic cells . The subunits of these complexes are called SNARE proteins. SNARE complexes catalyze at the Fusion of biological membranes transport of small molecules , for example at a exocytosis into the synaptic cleft.
properties
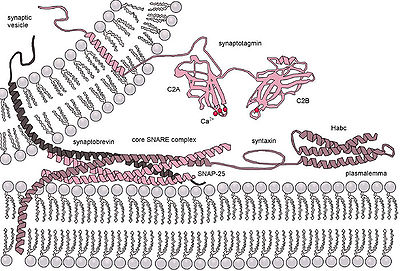
SNARE complexes are found in all secreting cells of eukaryotes . Nerve cells, for example, store their neurotransmitters fully synthesized in synaptic vesicles . If the transmitters are to be released outside the cell, the vesicle must fuse with the membrane and a pore must be formed through which the transmitter molecules can escape. The fusion and opening of the vesicle is controlled by SNARE and other proteins ( myosin II ).
The transport equation is:
- Neurotransmitter (vesicle) Neurotransmitter (extracellular)
Nine SNARE proteins are known in humans: synaptobrevin -1 and -2, syntaxin -1A and -4, snapin , SNAP-23 and -25, endobrevin and vesicle fusion protein NSF . At least a dozen different SNARE complexes are formed from three to five different of these proteins.
structure
The SNARE proteins form very stable complexes, which each consist of four parallel α-helices with a 60-70 amino acid SNARE motif, arranged in so-called “ coiled coils ” . The interior of this core complex (perpendicular to the twist axis) is made up of 16 layers of hydrophobic amino acids. An exception is the middle “0-layer”, which is formed by three glutamines (Q) and one arginine (R) - amino acids with polar side chains. Depending on their position in the core complex, a distinction is made between Q a , Q b , Q c and R-SNAREs, which are evolutionarily highly conserved. It is assumed that a functional SNARE complex must have the composition Q abc R. Some of the SNAREs are transmembrane proteins , of which at least one must be present in both of the membranes to be fused.
Task and function
Located on the vesicle surface (v-SNARE = vesicle synaptosome-associated protein receptor) and the target organelle (t-SNARE = target synaptosome-associated protein receptor), they ensure the correct occurrence of fusions, because each organelle or vesicle has a specific one Has SNARE composition. The fusion takes place in response to a molecular stimulus (e.g. increase in the Ca 2+ concentration with Synaptotagmin as sensor protein); During the fusion, water has to be displaced from the hydrophilic (water-loving) vesicle surface, which is extremely unfavorable in terms of energy and thus serves specificity, if namely only with the correct v- and t-SNARE combination sufficient energy is released through the protein interaction. In addition to the SNAREs, Rab proteins also play a role in the directed fusion of the vesicle and organelle.
The fusion that takes place then not only supplies the organelle with the contents of the vesicle, but also with new membrane lipids .
SNAREs as a target for neurotoxins
Tetanus toxin and botulinum toxin (Botox) play a role in blocking synapses. They split SNARE proteins, which prevents vesicle fusion and thus transmitter release . A tetanus spasm occurs when tetanus toxin blocks inhibitory synapses. There are seven known botulinum toxins, one of which is called Toxin A. This large protein is made up of two parts, with the shorter acting as an endopeptidase . This hydrolytically splits the SNAP-25 protein, which is located in the presynaptic membrane. Botox blocks z. B. excitatory synapses.
Individual evidence
- ↑ a b 1.F.1 The Synaptosomal Vesicle Fusion Pore (SVF-Pore) Family. In: TCDB. Saier Lab Bioinformatics, accessed September 8, 2010 .
- ↑ GO: 0031201 SNARE complex (children). In: Gene Ontology. EBI, accessed September 8, 2010 .