Hexokinase
| Hexokinase | ||
|---|---|---|

|
||
| Hexokinase from the yeast Kluyveromyces lactis | ||
| other names |
HK |
|
| Mass / length primary structure | 50 kDa in prokaryotes, 100 kDa in eukaryotes | |
| Isoforms | 4 in mammals | |
| Enzyme classification | ||
| EC, category | 2.7.1.1 , kinase | |
| Response type | Phosphorylation | |
| Substrate | Hexose + ATP | |
| Products | Hexose-6-phosphate + ADP | |
The hexokinases are enzymes from the carbohydrate metabolism that phosphorylate hexoses (sugars with six carbon atoms) and thus convert each into a hexose phosphate, such as glucose into glucose-6-phosphate . Hexokinases belong to the phosphotransferases ( EC 2.7).
properties
As kinases , the hexokinases lead to a phosphorylation of hexoses; glucose-6-phosphate is usually formed from glucose using adenosine triphosphate . The glucose-6-phosphate is then metabolized in the course of glycolysis or the pentose phosphate pathway .
Types of hexokinases
Hexokinases occur in all realms of living things and belong to the ATPases with actin fold . Hexokinases often come in several isoforms . There are four isoforms of hexokinase in mammals (I, II, III, and IV, or A, B, C, and D). The glucokinase in mammals is an isoform of hexokinase in the liver (hexokinase IV), but the only phosphorylated glucose. While bacterial hexokinases have a molar mass of around 50 kDa , eukaryotic hexokinases are around 100 kDa and are made up of two similar parts, which presumably have evolved from a precursor hexokinase through gene duplication . Both parts are enzymatically active only in hexokinase II.
Hexokinases are overexpressed in some tumors .
| Type | description | gene | Phenotypes |
|---|---|---|---|
| I (A) | occurs in all tissues of mammals and is considered a "household enzyme" that is unaffected by most physiological, hormonal and metabolic changes | HK1 | Charcot-Marie-Tooth disease type 4G, |
| II (B) | is the main regulated isoform in many cell types and is elevated in many types of cancer. It is the hexokinase that is found in muscles and the heart. Hexokinase II is also located on the outer mitochondrial membrane, so that direct access to ATP is possible. | HK2 | highly expressed in several cancers including breast cancer and colon cancer |
| III (C) | Is substrate-inhibiting in physiological concentrations due to glucose | HK3 | overexpressed in the malignant follicular thyroid nodule |
| IV (D) | occurs in the liver, pancreas, hypothalamus, small intestine and possibly in certain other neuroendocrine cells and plays an important regulatory role in carbohydrate metabolism. In the beta cells of the pancreatic islets, it serves as a glucose sensor to control the release of insulin and similarly to control the release of glucagon in the alpha cells. In hepatocytes of the liver, glucokinase responds to changes in ambient glucose levels by increasing or decreasing glycogen synthesis . | GCK | Maturity Onset Diabetes of the Young Type 2 , |
mechanism
Binding of glucose
From X-ray crystallographic studies of hexokinase from yeast it has been shown that the binding of glucose to the active site of a strong conformational change of the enzyme induced. The hexokinase consists of two lobes and when the glucose is bound, the lobes move towards each other. One lobe rotates twelve degrees relative to the other, which leads to movements of the polypeptide backbone of 0.8 nm. The closure of the gap in the hexokinase caused by Induced Fit ensures that the glucose is enveloped, except for the hydroxyl group on the C6 atom, to which a phosphoryl group is then transferred from the ATP. The structural changes induced by glucose have two advantages:
- The surroundings of the glucose become less polar, which favors the reaction with the hydrophilic hydroxyl group of glucose and the terminal phosphate group of ATP.
- The hexokinase pushes water out of the active center and ensures that water does not attack the γ-phosphate group of ATP. If the hydrolysis of ATP should occur, ADP and P i would arise .
The hexokinase is only active when Mg 2+ ions (or other divalent metal ions such as Mn 2+ ) are present.
Active site reaction
The hexokinase carries out an arbitrary “bi-bi” reaction mechanism in which the enzyme forms a ternary complex ( protein complex made up of three different molecules that are connected to each other) with glucose and Mg 2+ –ATP before the reaction . The hydroxyl group on the C6 atom of glucose carries out a nucleophilic attack on the γ-phosphate group of ATP and forms the ADP as a leaving group . This mechanism is also known as the S N 2 mechanism . The product is glucose-6-phosphate. The complexation of the Mg 2+ ion with ATP presumably serves to facilitate the nucleophilic attack on the phosphorus atom by shielding the negative charges on the oxygen atoms. The exact position (s) of the Mg 2+ ion in the complex with ATP during the reaction could not yet be finally determined.
regulation
Glucose-6-phosphate is a potent inhibitor of hexokinases 1–3 with an inhibition constant ( K i ) of 10–30 mM. There are two models that show the inhibitory effect of glucose-6-phosphate:
- As an allosteric inhibitor, glucose-6-phosphate binds firmly to the N -terminal domain in order to stabilize the closed conformation of the N -terminal domain. The closed N -terminal domain probably stabilizes the flexible subdomain of the C -terminal half of the enzyme (where the active center is also located) in such a way that the binding to ATP is prevented.
- Glucose-6-phosphate acts as a competitive inhibitor , which competes with ATP for the C -terminal domain as a binding site.
In the case of hexokinase 1, the inhibition by glucose-6-phosphate can be lifted again by physiological concentrations of P i . Fang et al . (1998) described two possible mechanisms for releasing the inhibition by P i :
- P i competes with glucose-6-phosphate for the allosteric binding site in the N -terminal half of the enzyme.
- P i binds to the N -terminal half of the enzyme and thus displaces glucose-6-phosphate from the active center in the C -terminal half via an indirect mechanism.
In both cases, binding to the N -terminal domain by P i regulates catalysis in the C -terminal region by an allosteric mechanism. Fang et al . also showed that the regulatory binding site for P i is located in the N -terminal domain, which also contains the low-affinity binding site for glucose-6-phosphate.
Glucokinase is not inhibited by glucose-6-phosphate. This enables continued signaling (e.g., to trigger insulin release) with significant levels of G6P.
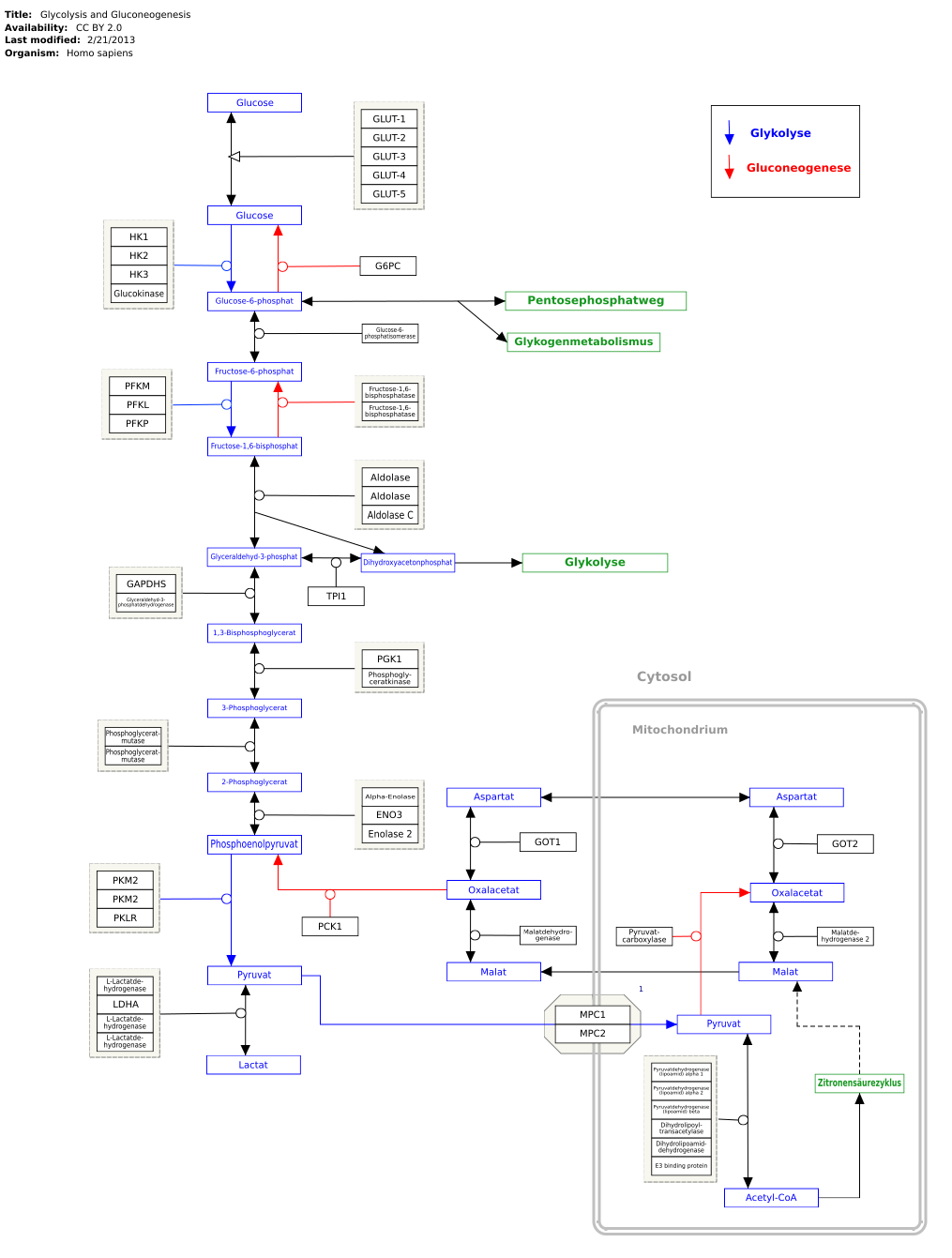
metabolism
Genes, proteins and metabolites are linked to the respective articles. The metabolic pathway can be edited at WikiPathways :
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3rd edition, John Wiley & Sons, New York 2004. ISBN 0-471-19350-X .
- Bruce Alberts , Alexander Johnson, Peter Walter, Julian Lewis, Martin Raff, Keith Roberts: Molecular Biology of the Cell , 5th Edition, Taylor & Francis 2007, ISBN 978-0-8153-4106-2 .
Individual evidence
- ↑ The following ENZYME entries belong to class 2.7.-.- ( English ) SIB Swiss Institute of Bioinformatics. Retrieved October 29, 2019.
- ↑ TA Smith: Mammalian hexokinases and Their abnormal expression in cancer. In: British journal of biomedical science. Volume 57, Number 2, 2000, ISSN 0967-4845 , pp. 170-178, PMID 10912295 .
- ↑ CMT4G. In: Online Mendelian Inheritance in Man . (English)
- ↑ LS Sullivan, DC Koboldt, SJ Bowne, S. Lang, SH Blanton, E. Cadena, CE Avery, RA Lewis, K. Webb-Jones, DH Wheaton, DG Birch, R. Coussa, H. Ren, I. Lopez , C. Chakarova, RK Koenekoop, CA Garcia, RS Fulton, RK Wilson, GM Weinstock, SP Daiger: A dominant mutation in hexokinase 1 (HK1) causes retinitis pigmentosa. In: Investigative ophthalmology & visual science. Volume 55, number 11, September 2014, pp. 7147-7158, doi : 10.1167 / iovs.14-15419 , PMID 25190649 , PMC 4224580 (free full text).
- ↑ F. Wang, Y. Wang, B. Zhang, L. Zhao, V. Lyubasyuk, K. Wang, M. Xu, Y. Li, F. Wu, C. Wen, PS Bernstein, D. Lin, S. Zhu, H. Wang, K. Zhang, R. Chen: A missense mutation in HK1 leads to autosomal dominant retinitis pigmentosa. In: Investigative ophthalmology & visual science. Volume 55, number 11, October 2014, pp. 7159-7164, doi : 10.1167 / iovs.14-15520 , PMID 25316723 , PMC 4224578 (free full text).
- ↑ UniProt P35354 # subcellular_location
- ^ A. Schindler, E. Foley: Hexokinase 1 blocks apoptotic signals at the mitochondria. In: Cellular signaling. Volume 25, Number 12, December 2013, pp. 2685-2692, doi : 10.1016 / j.cellsig.2013.08.035 , PMID 24018046 .
- ↑ D. Palmieri, D. Fitzgerald, SM Shreeve, E. Hua, JL Bronder, RJ Weil, S. Davis, AM Stark, MJ Merino, R. Kurek, HM Mehdorn, G. Davis, SM Steinberg, PS Meltzer, K Aldape, PS Steeg: Analyzes of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. In: Molecular cancer research: MCR. Volume 7, Number 9, September 2009, pp. 1438-1445, doi : 10.1158 / 1541-7786.MCR-09-0234 , PMID 19723875 , PMC 2746883 (free full text).
- ^ Q. Peng, J. Zhou, Q. Zhou, F. Pan, D. Zhong, H. Liang: Silencing hexokinase II gene sensitizes human colon cancer cells to 5-fluorouracil. In: Hepato-gastroenterology. Volume 56, Number 90, 2009 Mar-Apr, pp. 355-360, PMID 19579598 .
- ↑ Jeremy M. Berg, John L. Tymoczko, Lubert Stryer, Gregory J. Gatto, Jr.: Stryer Biochemistry . Springer-Verlag, 2014, ISBN 978-3-8274-2988-9 , pp. 460 ( limited preview in Google Book Search).
- ^ Donald Voet, Judith G. Voet: Biochemistry . 4th edition. John Wiley & Sons, 2010, ISBN 978-0-470-91745-9 , pp. 597 ( limited preview in Google Book search).
- ↑ AE Aleshin, C. Zeng, GP Bourenkov, HD Bartunik, HJ Fromm, RB Honzatko: The mechanism of regulation of hexokinase: new insights from the crystal structure of recombinant human brain hexokinase complexed with glucose and glucose-6-phosphate. In: Structure. Volume 6, Number 1, January 1998, pp. 39-50, doi : 10.1016 / s0969-2126 (98) 00006-9 , PMID 9493266 .
- ↑ DL Purich, HJ Fromm, FB Rudolph: The hexokinases: kinetic, physical, and regulatory properties. In: Advances in enzymology and related areas of molecular biology. Volume 39, 1973, pp. 249-326, doi : 10.1002 / 9780470122846.ch4 , PMID 4583639 (review).
- ↑ Tsuei-Yun Fang, Olga Alechina, Alexander E. Aleshin, Herbert J. Fromm, Richard B. Honzatko: Identification of a Phosphate Regulatory Site and a Low Affinity Binding Site for Glucose 6-Phosphate in the N-terminal Half of Human Brain Hexokinase. In: Journal of Biological Chemistry. 273, 1998, p. 19548, doi : 10.1074 / jbc.273.31.19548 .
- ^ GI Bell, A. Cuesta-Munoz, FM Matschinsky: Glucokinase . In: Encyclopedia of Molecular Medicine . John Wiley & Sons, Hoboken 2002, ISBN 978-0-471-37494-7 .
- ^ FM Matschinsky: Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. In: Diabetes. Volume 45, Number 2, February 1996, pp. 223-241, doi : 10.2337 / diab.45.2.223 , PMID 8549869 (review).