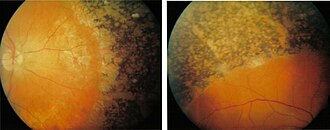
Retinopathia pigmentosa
| Classification according to ICD-10 | |
|---|---|
| H35.5 | Hereditary retinal dystrophy |
| ICD-10 online (WHO version 2019) | |
The term retinitis pigmentosa or Retinitis pigmentosa (RP) describes a through heredity or spontaneous mutation resulting retinal degeneration , in which the photoreceptors are destroyed. One speaks of pseudoretinitis pigmentosa (or phenocopy) if non-hereditary diseases show symptoms of retinopathia pigmentosa, for example due to toxicity (for example thioridazine , chloroquine ).
The name retinitis pigmentosa was coined by the Dutchman Frans Donders in 1855. Since this is not primarily an inflammation (-itis), the disease was renamed Retinopathia pigmentosa . The original name is still used synonymously and even more often than the new name.
The corresponding disease in animals is known in veterinary medicine as progressive retinal atrophy (PRA).
Epidemiology
Around three million people worldwide - around 30,000 to 40,000 in Germany - are affected by one of the various forms of retinopathia pigmentosa (also called Patermann syndrome). Due to the slow course of the disease, the number of unreported cases is likely to be even higher. The prevalence is thus one case per 3000 to 7000 inhabitants.
The disease usually occurs in adolescence or middle age with the first signs ( night blindness ); eyesight gradually deteriorates. The entire process of increasing visual impairment is insidious and usually extends over decades for those affected. This development is also associated with severe psychological stress. In about half of all RP patients, a cataract develops in adulthood .
Symptoms and course
The symptoms usually include:
- Night blindness (first clues)
- Tunnel vision , concentric restriction of field of vision (early signs)
- poor adaptation of the eye to changing light conditions
- Glare sensitivity
- Disturbance in contrast vision
- Impairment of color vision
- gradual loss of vision to complete blindness
In the course of the disease, night blindness, a drop in visual acuity and then a gradual restriction of the field of vision up to an increasingly narrowing tunnel vision occur . The disease usually leads to blindness at a later stage . Because of the night blindness and tunnel vision, patients can hardly move safely without using blind-specific orientation strategies and a long stick . The time course of this sequence varies, depending on the particular RP-triggering genetic defect .
The death of the photoreceptors , first of the rods , usually takes place from the periphery (the edge of the visual field) to the macula (the center of the visual field). Donders particularly described the pigment deposits and vascular constrictions in the eye, similar to bone bodies, which gave the retinopathia pigmentosa its name. However, these changes usually occur secondary to the degeneration of the photoreceptors.
genetics
Over 45 genes have now been identified whose defects can trigger retinopathia pigmentosa. However, so far only a little over half of all causative genetic factors have been identified. Most of the genes identified so far follow a monogenetic inheritance , i. H. the defect in just one gene causes the disease and not in several genes at the same time. The disease is triggered by autosomal dominant , autosomal recessive and gonosomal hereditary factors (mainly the X chromosome ).
Syndromes
Approximately 25% of the affected patients suffer from an associated retinopathia pigmentosa. In the case of associated RP, other organs of the body in addition to the eye also show symptoms of the disease, i.e. a syndrome is present. Some of these symptoms that often occur together with retinopathia pigmentosa are hearing disorders, paralysis and walking disorders, cardiac arrhythmias, muscle weakness, mental development disorders and the like. a. Here, too, genetic defects are the cause. The most famous syndromes are:
- the Usher syndrome ,
- the Bardet-Biedl syndrome ,
- the Refsum syndrome ,
- the NBIA syndrome ,
- the Alport syndrome
- the Saldino-Mainzer syndrome .
Diagnosis and treatment
Retinopathia pigmentosa can already be diagnosed in early childhood using an electroretinogram . Eye tests for night blindness at the ophthalmologist offer further diagnostic options . In the case of syndromes, the other symptoms provide information about the exact disease (such as hearing impairment or blood values). The determination of the exact genetic defect only enables a DNA analysis . DNA and protein chips , which should enable faster diagnosis, are still under development . This can also help with genetic family counseling.
There is currently no treatment that can prevent retinopathia pigmentosa from progressing or cure the disease. However, studies exist that the intake of vitamin A or anti-vascular endothelial growth factor should slow down the process. Another study shows that hyperbaric oxygen therapy can slow the course of the disease . Exceptions are special forms such as Refsum's syndrome, a metabolic defect in which a special diet low in phytanic acid or, if this is not sufficient, regular lipid apheresis can bring retinopathia pigmentosa to a standstill.
Still in the research are gene therapy approaches in which defective genes could be replaced in the retina, or stem cell therapies in which the degenerated retina to be repaired. So-called retina implants are also being developed , in which microsystem technology is used as a prosthesis to replace the functions of the defective retina.
Among other things, choroideremia is to be distinguished .
literature
- C. Hamel: Retinitis pigmentosa. In: Orphanet Journal of Rare Diseases. 1, 2006, p. 40. doi: 10.1186 / 1750-1172-1-40
- Aleksandra Polosukhina, Jeffrey Litt et al. a .: Photochemical Restoration of Visual Responses in Blind Mice. In: Neuron. 75, 2012, pp. 271–282, doi: 10.1016 / j.neuron.2012.05.022 .
Web links
- Pro Retina Germany e. V. Pro Retina Germany is a self-help association for people with retinal degeneration.
- Guideline No. 25 - Hereditary diseases of the retina, choroid or visual tract . (PDF) Professional Association of Ophthalmologists in Germany V. German Ophthalmological Society e. V.
Individual evidence
- ^ FC Donders: Contributions to the pathological anatomy of the eye. In: Arch for Ophthalmol. 1855-1, pp. 106-118.
- ↑ a b c S. P. Daiger u. a .: Perspective on genes and mutations causing retinitis pigmentosa. In: Arch Ophthalmol. 125 (2), Feb 2007, pp. 151-158. PMID 17296890
- ↑ a b D. T. Hartong et al. a .: retinitis pigmentosa. In: Lancet. 368 (9549) Nov 18, 2006, pp. 1795-1809. PMID 17113430
- ↑ MA Sandberg u. a .: Disease Course in Patients with Autosomal Recessive Retinitis Pigmentosa due to the USH2A Gene. In: Invest Ophthalmol Vis Sci. Jul 18, 2008, pp. 5532-5539. PMID 18641288 .
- ↑ a b P. Goodwin: Hereditary retinal disease. In: Curr Opin Ophthalmol. 19, (3), May 2008, pp. 255-262. PMID 18408503
- ↑ HBO therapy for retinitis pigmentosa
- ↑ K. Rüether, E. Baldwin, M. Casteels, MD Feher, M. Horn, S. Kuranoff, BP Leroy, RJ Wanders, AS Wierzbicki: Adult Refsum disease: a form of tapetoretinal dystrophy accessible to therapy. In: Surv Ophthalmol. 55 (6), 2010, pp. 531-538. PMID 20850855 .
- ↑ EJ Baldwin, FB Gibberd, C. Harley, MC Sidey, MD Feher, AS Wierzbicki: The effectiveness of long-term dietary therapy in the treatment of adult Refsum disease. In: J Neurol Neurosurg Psychiatry. 81 (9), 2010, pp. 954-957. PMID 20547622 .
- ^ I. Mooney, J. LaMotte: A review of the potential to restore vision with stem cells. In: Clin Exp Optom. 91 (1), Jan 2008, pp. 78-84. PMID 18045253
- ^ William A. Beltran, Artur V. Cideciyan et al. a .: Successful arrest of photoreceptor and vision loss expands the therapeutic window of retinal gene therapy to later stages of disease. In: Proceedings of the National Academy of Sciences. 112, 2015, pp. E5844 – E5853, doi: 10.1073 / pnas.1509914112 .
- ↑ AG Bassuk u. a .: Precision Medicine: Genetic Repair of Retinitis Pigmentosa in Patient-Derived Stem Cells. In: Sci. Rep. 6, 2016, p. 19969. doi: 10.1038 / srep19969
- ↑ N. Alteheld u. a .: Towards the bionic eye - the retina implant: surgical, ophthalmological and histopathological perspectives. In: Acta Neurochirurgica Suppl. 97 (Pt 2), 2007, pp. 487-493. PMID 17691339 .