Chloroquine
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) |
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Chloroquine | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 18 H 26 ClN 3 | ||||||||||||
| Brief description |
white to almost white, crystalline, hygroscopic powder (diphosphate) |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | 319.87 g · mol -1 | ||||||||||||
| Melting point |
|
||||||||||||
| boiling point |
214–221 ° C (27 Pa, free base) |
||||||||||||
| pK s value |
|
||||||||||||
| solubility | |||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Chloroquine , trade name Resochin , is a mixture of two enantiomeric chemical compounds , some of which are structurally related to quinine . Hydroxychloroquine is an analog preparation to chloroquine .
Chloroquine and hydroxychloroquine can be used as drugs for therapy and chemoprophylaxis of malaria . However, widespread pathogen resistance severely restrict the application for these indications . In addition, chloroquine is used as an agent in the treatment of rheumatic diseases such as lupus erythematosus and rheumatoid arthritis , porphyria cutanea tarda and the rare form of extraintestinal amoebiasis .
history
Chloroquine was synthesized in 1934 by Hans Andersag at IG Farbenindustrie in Elberfeld. However, this agent called Resochin was initially of no importance, since the German Wehrmacht used the closely related Sontochin (methylated chloroquine), which was invented at the same time, for malaria prophylaxis during World War II . Samples from Sontochin were found in German prisoners of war in North Africa and analyzed in the USA. Analogous substances were also examined there, showing the superior effect and tolerability of chloroquine in comparison to other drugs such as atebrine , the malaria drug used by the Allies in the Pacific War under the name of quinacrine. After the Second World War, chloroquine was a highly effective agent for a long time; In the meantime, many malaria parasites have developed resistance to the drug.
In July 2019, Bayer AG stopped selling its Resochin product . It was the only product available in Germany with the active ingredient chloroquine phosphate. According to the company, it was no longer possible to manufacture the drug in the required quality. However, Bayer continued to sell the product in Pakistan , where, according to the company, production of Resochin was " restarted" as part of the 2020 COVID-19 pandemic . In the event that it is effective against COVID-19, the German government has reserved “larger quantities” of the drug at Bayer.
presentation
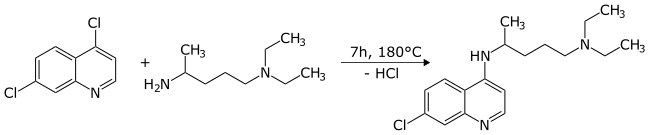
In 1937 IG Farben patented the synthesis of chloroquine by reacting 4,7-dichloroquinoline with 1-diethylamino-4-aminopentane :
properties
Chloroquine is a colorless to yellowish, odorless and almost water-insoluble compound. Due to the basic properties of the substance, it dissolves well in acids . The much more water-soluble salts chloroquine diphosphate (50 g / l) and chloroquine sulfate are used as medicinal substances . The free base decomposes on contact with daylight and atmospheric oxygen; the salts are mostly stable in air.
Analytics
Reliable qualitative and quantitative determination in various test materials is possible after appropriate sample preparation by coupling HPLC with mass spectrometry .
Mode of action
Chloroquine inhibits the crystallization of hemozoin , a breakdown product of heme . Hemozoin is produced when the malaria pathogen breaks down the hemoglobin in infected red blood cells in order to obtain proteins for its metabolism. If the hemozoin can no longer be crystallized, this leads to the death of the parasite.
Chloroquine has a blood schizontocidal effect, that is, it leads to an inhibition in the later erythrocytic stage of the pathogen. Chloroquine was once the most widely used drug in the world for the therapy and prevention of malaria, but it is now increasingly ineffective due to resistant pathogens.
Medical use
malaria
Therapy regimen
As a preventive measure against malaria, the recommended (oral) dose for adults is 500 mg chloroquine phosphate per week, starting 1 to 2 weeks before departure and continuing up to four weeks after the end of the journey. Due to the frequent resistance, chloroquine was often used in combination with other active ingredients, especially proguanil . Effectiveness and tolerance are mostly rated negatively. The combination is therefore usually no longer common in German-speaking countries today - also due to the existence of alternatives with a better risk-benefit ratio. Exceptions to this are considered in children and during pregnancy. Chloroquine requires a prescription. In the case of chloroquine poisoning, diazepam can be administered i.v. be used as an antidote . The biological half-life of the substance increases significantly with continued intake of initially a few days due to considerable accumulation in the tissue (up to 30-60 days; according to other sources, to 40 to 50 days or two to three weeks.)
Side effects and contraindications
Chloroquine can have various side effects, including clouding of the cornea and changes in the retina in the eye , gastrointestinal complaints, sleep disorders, neuropsychiatric symptoms and reddened skin. Chloroquine must not be used, particularly in the case of severe liver and kidney damage . The combination of chloroquine together with drugs that damage the liver or MAO inhibitors (see monoamine oxidase inhibitors ) must not be used. Chloroquine must not be used if there are diseases of the eye and the blood-forming system. If there is a hypersensitivity to quinine or mefloquine , chloroquine must not be used for therapy.
In some patients, chloroquine can cause a dose-dependent prolongation of the QT interval . In patients with pre-existing cardiac diseases or with simultaneous use of substances that prolong the QT interval, the risk of cardiac arrhythmias - sometimes with fatal outcome - is increased. An overdose of the drug was reported in 2012 by the association SterbeHilfeDeutschland e. V. used as a means of killing in the context of euthanasia .
The weekly intake of chloroquine is permitted for pregnant women and nursing mothers, but should be avoided for long-term use with daily intake (as in the treatment of rheumatoid arthritis). Otherwise there is a risk of eye defects in the unborn or infant. Chloroquine can also be used in children, but long-term treatment should not be used.
Overdosing leads to pigmentation disorders that make hair bleach.
According to a study from 2008, chloroquine has also been linked to inducing antibiotic resistance (to fluoroquinolones ).
In the WHO Pharmaceutical Newsletter No. 6/2019 , the occurrence of Stevens-Johnson syndrome and Lyell syndrome are described as serious side effects .
Rheumatic form circle
Chloroquine is also used for the therapy of rheumatoid arthritis . Severe courses of Porphyria cutanea tarda are treated with low-dose chloroquine or hydroxychloroquine (for example 200 mg twice a week).
COVID-19
After chloroquine had already shown effectiveness against the first SARS coronavirus in an in vitro study in cell culture , it was considered for the treatment of COVID-19 . Here, too, promising results were shown in cell culture with a mean effective concentration in the micromolar range for both chloroquine and hydroxychloroquine.
A review from March 10, 2020 concludes that high quality clinical data from different geographic regions is urgently needed. The Institute for Tropical Medicine at the Tübingen University Hospital announced an application for approval for a clinical study on March 18, 2020 . This was advocated by the Paul Ehrlich Institute and the Federal Institute for Drugs and Medical Devices (BfArM). The study, in which the Bernhard Nocht Institute for Tropical Medicine is also involved, is to take place in a placebo-controlled manner and begin on March 23, 2020. Further clinical trials have been announced by the University of Oxford and the University of Queensland . On March 20, 2020, the World Health Organization started the SOLIDARITY study, which aims to evaluate chloroquine and other active ingredients on thousands of patients worldwide.
A short scientific article from China reported good experiences in treating COVID-19 patients with chloroquine, but did not quantify these results. In contrast, a clinical pilot study carried out in Shanghai found no advantage for hydroxychloroquine over conventional treatment . A clinical study by a hospital in Marseille came to positive results with the use of hydroxychloroquine, but was widely criticized for its methodology. Based on some data, the BfArM sees a possible benefit of chloroquine, especially if it is used early.
Chloroquine and hydroxychloroquine are recommended in the Belgian provisional treatment guidelines and the South Korean guidelines for COVID-19 patients.
In the United States, the drug attracted a lot of public attention after President Donald Trump expressed high expectations of its effectiveness at a press conference and in a tweet . And even if it shouldn't be effective, it won't kill anyone (Trump on March 19, 2020: " But the nice part is, it's been around for a long time, so we know that if it - if things don't go as planned, it's not going to kill anybody. ").
Stephen Hahn, head of the Food and Drug Administration (FDA), warned that the drug may do more harm than good. In France, people died after uncontrolled self-administration as a result of serious interactions with other drugs. As the US Department of Health announced on March 30, 2020, the FDA had issued an Emergency Use Authorization (EUA) that allows the dispensing or prescription of chloroquine and hydroxychloroquine in emergencies "by doctors to adolescent and adult patients with Covid in hospital -19 “and revoked it on June 15, 2020. The European Medicines Agency said on 1 April 2020 that patients and healthcare professionals chloroquine and hydroxychloroquine may use only for their approved applications, or likely to be used but maximum in clinical trials or national emergency treatment programs for COVID-19th
In Brazil, a clinical study began on March 23, 2020 in a hospital in Manaus . In the double-blind , randomized , adaptive, two-arm phase IIb study , one group of Covid-19 patients received 600 mg of chloroquine twice daily for ten days (high dose) and the other group received 450 mg once daily for five days with a double dose Day 1. A total of 440 people should be enrolled in the study. However, after 81 patients were enrolled, the high-dose arm of the study was stopped after several of those patients suffered fatal arrhythmias or cardiac muscle damage.
On April 24, 2020, the FDA issued a Drug Safety Communication warning of complications that may be associated with the use of chloroquine and hydroxychloroquine. There have been reports of serious heart rhythm problems in Covid 19 patients treated with these substances. Those affected were often treated in combination with azithromycin or other QT-prolonging drugs. When using hydroxychloroquine or chloroquine in the context of emergency programs or clinical studies, she recommended an initial evaluation and monitoring of the therapy with, for example, ECG , electrolytes, as well as kidney function and liver tests.
A study by the Irving Medical Center in Manhattan published on May 7th in The New England Journal of Medicine came to the conclusion that hydroxychloroquine is neither harmful nor beneficial in Covid-19 diseases. The drug was used in 811 patients (control group: 565 patients), which makes it supposedly the largest series of treatments in the world to date. According to the Deutsches Ärzteblatt , the experience "will probably lead to hydroxychloroquine no longer being used to treat COVID-19."
Medicinal products containing chloroquine and hydroxychloroquine are part of a series of drugs, of which the German Federal Ministry of Health will purchase millions of packs from April 2020 to be used as an alternative in the event of severe courses against Covid-19.
Biochemical use and importance
Chloroquine is also used in cell culture for transfection used to increase the efficiency of transfection. It inhibits the lysosomal DNases by neutralizing the pH within the vesicles . Chloroquine inhibits also the endocytosis . Since it also inhibits autophagy , it is being discussed as a potential chemotherapeutic agent . Clinical studies are carried out worldwide with chloroquine in combination with classic chemotherapeutic agents.
Use in the aquarium hobby
Outside of human medicine, chloroquine phosphate is recommended to combat certain parasites in aquarium fish . The FDA recently warned against the human medical use of these products after a serious illness and death became known in the United States.
Individual evidence
- ↑ a b European Pharmacopoeia Commission (ed.): EUROPEAN PHARMACOPOE 5TH EDITION . tape 5.0-5.8 , 2006.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-114.
- ↑ a b c d e f g Beate Blümer-Schwinum, Hermann Hager, Franz von Bruchhausen, E. Nuremberg, Peter Surmann: Hagers Handbook of Pharmaceutical Practice. Volume 7: Fabrics A – D. 5th edition. Birkhäuser, 1995, ISBN 3-540-52688-9 , pp. 884-891.
- ↑ a b c data sheet Chloroquine diphosphate salt from Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ↑ Entry on chloroquine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Recommendations for the prevention of malaria. ( Page no longer available ) German Society for Tropical Medicine and International Health e. V., May 2016.
- ↑ German Society for Tropical Medicine and International Health e. V .: Guideline Diagnostics and Therapy of Malaria. ( Memento of the original from March 20, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. October 2015. AWMF guidelines register 042/001.
- ↑ W. Sneader: Drug Discovery. A history. Wiley, 2005, ISBN 0-471-89980-1 .
- ↑ Alexandra Negt: Bayer: Resochin goes out of distribution. Apotheke Adhoc, November 1, 2019.
- ↑ Corona virus: An active ingredient from Bayer could help. In: Bayer Magazine. Bayer AG, accessed March 23, 2020 .
- ↑ Rasmus Buchsteiner: Spahn secures Germany possible anti-corona drug. Editorial network Germany, March 18, 2020, accessed on March 20, 2020 .
- ↑ Patent DE683692A : Process for the preparation of quinoline compounds containing amino groups with basic substituents in position 4. Registered on October 8, 1937 , published on November 13, 1939 , applicant: IG Farbenindustrie Akt.-Ges. in Frankfurt, Main, inventors: Hans Andersag, Stefan Breitner, Heinrich Jung.
- ↑ Karnrawee Kaewkhao, Kesinee Chotivanich, Markus Winterberg, Nicholas PJ Day, Joel Tarning, Daniel Blessborn: High sensitivity methods to quantify chloroquine and its metabolite in human blood samples using LC-MS / MS , bioanalysis. 2019 Mar; 11 (5): 333-347, PMID 30873854
- ↑ Hence A, Aljayyoussi G, Pereira D, Lacerda MVG, Alexandre MAA, Nascimento CT, Alves JC, da Fonseca LB, da Silva DMD, Pinto DP, Rodrigues DF, Silvino ACR, de Sousa TN, de Brito CFA, Ter Kuile FO , Lalloo DG: Pharmacokinetics / pharmacodynamics of chloroquine and artemisinin-based combination therapy with primaquine. , Malar J. 2019 Sep 23; 18 (1): 325, PMID 31547827
- ↑ Malaria prophylaxis after the mefloquine (Lariam) withdrawal. In: Medicinal Telegram. Vol. 47, No. 6, 2016, pp. 57–59.
- ↑ P. Schlagenhauf, A. Tschopp, R. Johnson, HD Nothdurft, B. Beck, E. Schwartz, M. Herold, B. Krebs, O. Veit, R. Allwinn, R. Steffen: Tolerability of malaria chemoprophylaxis in non -immune travelers to sub-Saharan Africa: multicentre, randomized, double blind, four arm study. In: BMJ. 327 (7423), Nov 8, 2003, p. 1078. PMC 261741 (free full text).
- ↑ Recommendations for the prevention of malaria . German Society for Tropical Medicine and International Health V., April 2011.
- ↑ Recommendations for the prevention of malaria . ( Page no longer available ) German Society for Tropical Medicine and International Health e. V., May 2016 (PDF file).
- ↑ Malaria - RKI Ratgeber , Robert Koch Institute, as of 2015, accessed April 21, 2018.
- ↑ Antidottarium the Red List .
- ↑ Chloroquine at Toxinfo.org ( Memento from November 30, 2010 in the Internet Archive ), Toxicological Department of the II. Medical Clinic of the Technical University of Munich .
- ^ Hydroxychloroquine - Chloroquine according to: Goodman & Gilman's: The Pharmacological Basis of Therapeutics. 9th edition, 1996.
- ↑ Resochin tablets / Resochin junior tablets. (PDF; 149 kB) Technical information from Bayer AG from September 2004.
- ↑ Inflammatory joint and spinal diseases: perioperative drug management. ( Memento of the original from July 17, 2011 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved April 11, 2013.
- ^ A. Knobloch: Therapy of uncomplicated tropic malaria with chloroquine and sulfadoxine-pyrmethamine in children in Tamale, Ghana. (PDF; 559 kB) Dissertation. University Medicine Berlin, 2005.
- ↑ Heimann, Heinrich et al .: Atlas of the fundus. Chapter 7: Macular Disorders , Section 7.7: Chloroquine and Hydroxychloroquine Retinopathy . Thime, 2010. doi : 10.1055 / b-0034-40509 .
- ↑ Richard Bergholz: Chloroquine Maculopathy: Risk Factors and Early Detection. Medical habilitation thesis. Medical Faculty Charité - Universitätsmedizin Berlin, November 2017. [1]
- ^ NM Maxwell, RL Nevin, S. Stahl, J. Block, S. Shugarts, AH Wu, S. Dominy, MA Solano-Blanco, S. Kappelman-Culver, C. Lee-Messer, J. Maldonado, AJ Maxwell: Prolonged neuropsychiatric effects following management of chloroquine intoxication with psychotropic polypharmacy. In: Clinical case reports. Volume 3, Number 6, June 2015, pp. 379-387, doi: 10.1002 / ccr3.238 . PMID 26185633 , PMC 4498847 (free full text).
- ^ RL Nevin, AM Croft: Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. In: Malaria journal. Volume 15, 2016, p. 332, doi: 10.1186 / s12936-016-1391-6 . PMID 27335053 , PMC 4918116 (free full text) (review).
- ↑ Resochin® tablets 250 mg, Resochin® junior tablets 81 mg. (PDF) Bayer Vital GmbH, February 2019, accessed on April 17, 2020 .
- ↑ Warning against premature use of chloroquine-azithromycin combination therapy against COVID-19 infections: risk of malignant cardiac arrhythmias. In: herzstiftung.de. German Heart Foundation , March 25, 2020, accessed on April 23, 2020 .
- ↑ Hamburg public prosecutor's office is bringing charges against the former Justice Senator Dr. Kush. Press portal, Hamburg Public Prosecutor's Office (2014) STA-HH.
- ↑ Nezile Mthembu: Science Shot: Anti-Malaria Drug bleaches hair . ( Memento of the original from July 23, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. In: Science. July 21, 2010.
- ↑ Ross J. Davidson, Ian Davis, Barbara M. Willey, Keyro Rizg, Shelly Bolotin, Vanessa Porter, Jane Polsky, Nick Daneman, Allison McGeer, Paul Yang, Dennis Scolnik, Roy Rowsell, Olga Imas, Michael S. Silverman: Antimalarial Therapy Selection for Quinolone Resistance among Escherichia coli in the Absence of Quinolone Exposure, in Tropical South America . In: PLOS ONE . tape 3 , no. 7 , July 16, 2008, doi : 10.1371 / journal.pone.0002727 .
- ↑ | WHO Pharmaceutical Newsletter No. 6/2019
- ↑ T. Cainelli, C. Di Padova et al. a .: Hydroxychloroquine versus phlebotomy in the treatment of porphyria cutanea tarda. In: British Journal of Dermatology . Volume 108, Number 5, May 1983, pp. 593-600. PMID 6849826 .
- ↑ Els Keyaerts, Leen Vijgen, Piet Maes, Johan Neyts, Marc Van Ranst: In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine . In: Biochemical and Biophysical Research Communications . tape 323 , no. 1 , October 8, 2004, doi : 10.1016 / j.bbrc.2004.08.085 .
- ↑ Manli Wang, Ruiyuan Cao, Leike Zhang, Xinglou Yang, Jia Liu, Mingyue Xu, Zhengli Shi, Zhihong Hu, Wu Zhong, Gengfu Xiao: Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro . Letter to the Editor. In: Cell Research . tape 30 , February 4, 2020, doi : 10.1038 / s41422-020-0282-0 .
- ↑ Xueting Yao, Fei Ye, Miao Zhang, Cheng Cui, Baoying Huang, Peihua Niu, Xu Liu, Li Zhao, Erdan Dong, Chunli Song, Siyan Zhan, Roujian Lu, Haiyan Li, Wenjie Tan, Dongyang Liu: In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) . In: Clinical Infectious Diseases . March 9, 2020, doi : 10.1093 / cid / ciaa237 ( forthcoming , pre-release).
- ↑ Jia Liu, Ruiyuan Cao, Mingyue Xu, Xi Wang, Huanyu Zhang, Hengrui Hu, Yufeng Li, Zhihong Hu, Wu Zhong, Manli Wang: Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro . In: Cell Discover . tape 6 , March 18, 2020, doi : 10.1038 / s41421-020-0156-0 .
- ↑ Andrea Cortegiani, Giulia Ingoglia, Mariachiara Ippolito, Antonino Giarratano, SharonEinav: A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19 . In: Journal of Critical Care . March 10, 2020, doi : 10.1016 / j.jcrc.2020.03.005 ( forthcoming, advance publication).
- ↑ Tropical doctors in Tübingen want test on corona drug. In: swr.de. March 17, 2020, accessed March 18, 2020 .
- ↑ Jonas Schmidt-Chanasit, Virologist Bernhard Nocht Institute for Tropical Medicine estimates. In: Topics of the day. March 22, 2020, accessed on March 23, 2020 (video interview).
- ↑ Georgia Tscharke: Active ingredient chloroquine against corona disease in the test. In: BR24. Bayerischer Rundfunk, March 19, 2020, accessed on March 20, 2020 .
- ↑ Chloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting (COPCOV). In: ClinicalTrials.gov. US National Library of Medicine, March 11, 2020, accessed March 23, 2020 ( study registration ).
- ^ Sarah McPhee: 'Cure' found for coronavirus in Australia. In: The Chronicle. March 16, 2020, accessed March 23, 2020 .
- ↑ Kai Kupferschmidt, Jon Cohen: WHO launches global megatrial of the four most promising coronavirus treatments. In: Science. March 22, 2020, accessed March 23, 2020 .
- ↑ Jianjun Gao, Zhenxue Tian, Xu Yan: Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies . Letter. In: BioScience Trends . tape 14 , no. 1 , February 29, 2020, doi : 10.5582 / bst.2020.01047 .
- ↑ Chen Jun, Liu Danping, Liu Li, Liu Ping, Xu Qingnian, Xia Lu, Ling Yun, Huang Dan, Song Shuli, Zhang Dandan, Qian Zhiping, Li Tao, Shen Yinzhong, Lu Hongzhou: A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) . In: Journal of Zhejiang University (Medical Sciences) . tape 49 , no. 1 , March 3, 2020, doi : 10.3785 / j.issn.1008-9292.2020.03.03 ( com.cn [PDF]).
- ↑ Philippe Gautret, Jean-Christophe Lagier, Philippe Parola, Van Thuan Hoang, Line Meddeb, Morgane Mailhe, Barbara Doudier, Johan Courjon, Valérie Giordanengo, Vera Esteves Vieira, Hervé Tissot Dupont, Stéphane Honoré, Philippe Colson, Eric Chabrière, Bernard La Scola, Jean-Marc Rolain, Philippe Brouqui, Didier Raoult: Hydroxychloroquine and azithromycin as a treatment of COVID ‐ 19: results of an open ‐ label non ‐ randomized clinical trial . In: International Journal of Antimicrobial Agents . doi : 10.1016 / j.ijantimicag.2020.105949 .
- ↑ Hanno Böck: About that Hydroxychloroquine for COVID-19 trial. In: Better Science. March 23, 2020, accessed March 23, 2020 .
- ↑ Anja Martini, Christian Drosten : Coronavirus Update. (PDF) Episode 22. In: ndr.de. Norddeutscher Rundfunk, March 26, 2020, p. 4 f. , accessed on March 27, 2020 .
- ↑ a b Ulla Thiede: Do we have a drug against Covid-19 soon? In: general-anzeiger-bonn.de. April 10, 2020, accessed April 13, 2020 .
- ↑ Instituut voor Tropische Geneeskunde, University of Antwerp, UMC Sint-Pieter, Sciensano, Federaal Agentschap voor Geneesmiddelen en Gezondheidsproducten (Ed.): Interim Clinical Guidance for Patients Suspected of / Confirmed with COVID-19 in Belgium . Version 4. March 19, 2020 ( wiv-isp.be [PDF; accessed March 23, 2020]).
- ↑ Kwak Sung-sun: Physicians work out treatment guidelines for coronavirus. In: Korea Biomedical Review. February 13, 2020, accessed March 23, 2020 .
- ↑ Riley Beggin: Trump keeps promoting an unproven coronavirus treatment - despite his experts' advice. In: Vox.com. Vox Media, March 21, 2020, accessed March 23, 2020 .
- ^ Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing. The White House, March 19, 2020, accessed April 23, 2020 (American English).
- ↑ Tara Haelle: Man Dead From Taking Chloroquine Product After Trump Touts Drug For Coronavirus. In: forbes.com. March 23, 2020, accessed on March 31, 2020 .
- ↑ America approves anti-malarial drugs for coronavirus. In: faz.de. March 30, 2020, accessed March 31, 2020 .
- ^ Letter of Authorization. FDA, March 28, 2020, accessed March 31, 2020 . Available at: Emergency Use Authorization. FDA, accessed March 31, 2020 .
- ↑ Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. US Food and Drug Administration (FDA), June 15, 2020, accessed June 16, 2020 .
- ↑ COVID-19: chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programs. In: ema.europea.eu. European Medicines Agency , April 1, 2020, accessed April 3, 2020 .
- ↑ Helga Blasius: Brazilian study stops high-dose administration of chloroquine. In: Deutsche Apotheker Zeitung. April 16, 2020, accessed April 23, 2020 .
- ↑ COVID-19: Smaller study with chloroquine canceled due to complications. In: Deutsches Ärzteblatt. April 14, 2020, accessed April 23, 2020 .
- ↑ Deutsche Welle: Several studies show: chloroquine increases the risk of death with COVID-19 | DW | 04/22/2020. Retrieved April 23, 2020 .
- ↑ Mayla Gabriela Silva Borba, Fernando Fonseca Almeida Val, Vanderson Souza Sampaio, Marcia Almeida Araújo Alexandre, Gisely Cardoso Melo, Marcelo Brito, Maria Paula Gomes Mourão, José Diego Brito-Sousa, Djane Baía-da-Silva, Marcus Vinitius Farias Guerra, Ludhmila Abrahão Hajjar, Rosemary Costa Pinto, Antonio Alcirley Silva Balieiro, Felipe Gomes Naveca, Mariana Simão Xavier, Alexandre Salomão, André Machado Siqueira, Alexandre Schwarzbolt, Júlio Henrique Rosa Croda, Maurício Lacerda Nogueira, Gustavo Adolfo Sierra Romero, Jesus Bassat, Jesus Bass Fontes, Bernardino Cláudio Albuquerque, Cláudio Tadeu Daniel-Ribeiro, Wuelton Marcelo Monteiro, Marcus Vinícius Guimarães Lacerda, and CloroCovid-19 Team: Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS -CoV-2) infection: Preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study). In: medRxiv preprint. April 11, 2020, accessed on April 23, 2020 .
- ↑ FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems , FDA, April 24, 2020.
- ↑ Neil W. Schluger, MD et al .: Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19 . In: Massachusetts Medical Society (Ed.): New England Journal of Medicine . May 7, 2020, ISSN 0028-4793 , doi : 10.1056 / NEJMoa2012410 , PMID 32379955 , PMC 7224609 (free full text) - ( nejm.org [accessed May 19, 2020]).
- ↑ Deutscher Ärzteverlag GmbH, editorial office of the Deutsches Ärzteblatt: COVID-19: In a study, hydroxychloroquine again proves to be ... May 12, 2020, accessed on May 19, 2020 .
- ↑ Kazuhiro Nogi: What can the flu drug Avigan, which the federal government is buying, do? In: spiegel.de. April 2, 2020, accessed April 2, 2020 .
- ↑ R. Delvecchio, LM Higa et al. a .: Chloroquine, an Endocytosis Blocking Agent, Inhibits Zika Virus Infection in Different Cell Models. In: Viruses. Volume 8, number 12, November 2016, p., Doi : 10.3390 / v8120322 , PMID 27916837 , PMC 5192383 (free full text).
- ↑ Y. Zhang, Z. Liao et al. a .: The utility of chloroquine in cancer therapy. In: Current medical research and opinion. Volume 31, number 5, May 2015, pp. 1009-1013, doi : 10.1185 / 03007995.2015.1025731 , PMID 25734693 (review).
- ^ VR Solomon, H. Lee: Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. In: European journal of pharmacology. Volume 625, number 1-3, December 2009, pp. 220-233, doi : 10.1016 / j.ejphar.2009.06.063 , PMID 19836374 (review).
- ^ JM Levy, CG Towers, A. Thorburn: Targeting autophagy in cancer. In: Nature Reviews Cancer . Volume 17, number 9, September 2017, pp. 528-542, doi : 10.1038 / nrc.2017.53 , PMID 28751651 (review).
- ↑ CG Towers, A. Thorburn: Therapeutic Targeting of Autophagy. In: EBioMedicine. Volume 14, December 2016, pp. 15-23, doi : 10.1016 / j.ebiom.2016.10.034 , PMID 28029600 , PMC 5161418 (free full text) (review).
- ^ Edward J. Noga: Fish Disease - Diagnosis and Treatment . 2nd Edition. Wiley-Blackwell, 2010, ISBN 978-0-8138-0697-6 , pp. 390 f .
- ↑ [2]
Trade names
Chloroquin (CH), Nivaquine (CH), Resochin (D, A), Weimer quin (D)