Mefloquine
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Mefloquine: 1: 1 stereoisomeric mixture of ( S , R ) -form (left) ( R , S ) -form (right) | ||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Mefloquine | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 17 H 16 F 6 N 2 O | |||||||||||||||
| Brief description |
white to pale yellow, crystalline and polymorphic powder (hydrochloride) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| pK s value |
8.6 (hydrochloride) |
|||||||||||||||
| solubility |
very sparingly soluble in water, slightly soluble in methanol , soluble in ethanol 96% (HCl) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Mefloquine is a synthetically produced drug for the prophylaxis and therapy of malaria . The drug is a joint development of the Walter Reed Army Institute of Research (WRAIR) of the United States Army and the pharmaceutical company F. Hoffmann-La Roche AG . It is subject to a doctor's prescription and may only be prescribed in Germany and numerous other countries due to possible serious and long-lasting side effects after completing a checklist for contraindications and handing out a patient passport.
Mefloquine is effective against intraerythrocytic asexual forms of the malaria pathogens Plasmodium falciparum , P. vivax , P. malariae , P. ovale in humans. ( Pathogens of tropical malaria , M. tertiana , M. quartana ). In particular, mefloquine is also effective against malaria parasites, which have developed resistance to other anti-malarials such as chloroquine , proguanil , pyrimethamine and pyrimethamine-sulfonamide combinations.
Mefloquine interrupts one of the most important metabolic functions of the malaria pathogens, as a result of which the pathogens slowly die off. However, the exact mechanism of action is unknown. The active ingredient is structurally related to quinine and chloroquine.
In some areas of Southeast Asia there is often resistance to Plasmodium falciparum , which is why other active ingredients must be used in the event of an infection. In the event of infection with the Plasmodium vivax pathogen , further treatment with another drug is recommended in order to prevent recurrences (see also: primaquine ).
It is generally advised not to use mefloquine for treatment if it has already been taken as a chemoprophylaxis .
From the German Society for Tropical Medicine and Global Health e. V. (DTG), the drug is currently recommended as an alternative to atovaquone-proguanil or doxycycline for the prophylaxis of tropical malaria only if there is a justified medical indication and in compliance with the special warnings in areas with a high risk of transmission without mefloquine-resistant pathogens.
The use of mefloquine for the treatment of malaria (both for emergency self-treatment and under inpatient conditions ) is normally no longer recommended because of possible serious side effects.
In Germany, the then manufacturer Roche waived the approval of Lariam , the only mefloquine preparation on the German market, in February 2016 . Sales ceased in April of the same year. The German wholesalers and pharmacies were allowed to sell off remaining stocks for another two years. In addition, parallel imports were available on the German market, the use of which was explicitly recommended by the DTG.
In May 2020 it was announced that the reference authorization for the Lariam products of the companies Emra-Med Arzneimittel GmbH (PZN: 07393184) and kohlpharma (PZN: 08898302) has expired and the Federal Institute for Drugs and Medical Devices (BfArM) is revoking the approval has arranged. The companies concerned then recalled all batches with immediate effect. In June, all batches of EurimPharm Arzneimittel GmbH (PZN: 08860647) were recalled and in July Orifarm (PZN: 06194100) and Pharma Gerke Arzneimittelvertriebs GmbH (PZN: 08859087) were recalled. This revocation of the approval of mefloquine parallel imports by the BfArM was reported elsewhere in the same month.
chemistry
Stereoisomerism
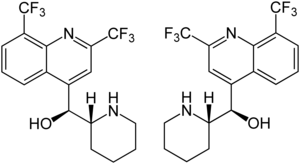
Mefloquine contains two stereogenic centers, therefore there are four stereoisomers:
- ( A ) ( S ) - (2,8-bis (trifluoromethyl) quinolin-4-yl) (( R ) -piperidin-2-yl) methanol - [(+) - erythro -Mefloquine]
- ( B ) ( R ) - (2,8-bis (trifluoromethyl) quinolin-4-yl) (( S ) -piperidin-2-yl) methanol - [(-) - erythro -Mefloquine]
- ( C ) ( R ) - (2,8-bis (trifluoromethyl) quinolin-4-yl) (( R ) -piperidin-2-yl) methanol - [(-) - threo -Mefloquine]
- ( D ) ( S ) - (2,8-bis (trifluoromethyl) quinolin-4-yl) (( S ) -piperidin-2-yl) methanol - [(+) - threo -Mefloquine]
The drug used is the racemate [1: 1 mixture] composed of the first two stereoisomers mentioned , which are enantiomeric to one another . The other two stereoisomers [( R , R ) form and ( S , S ) form] are diastereomers of the racemic drug [( S , R ) form and ( R , S ) form] and have no practical meaning.
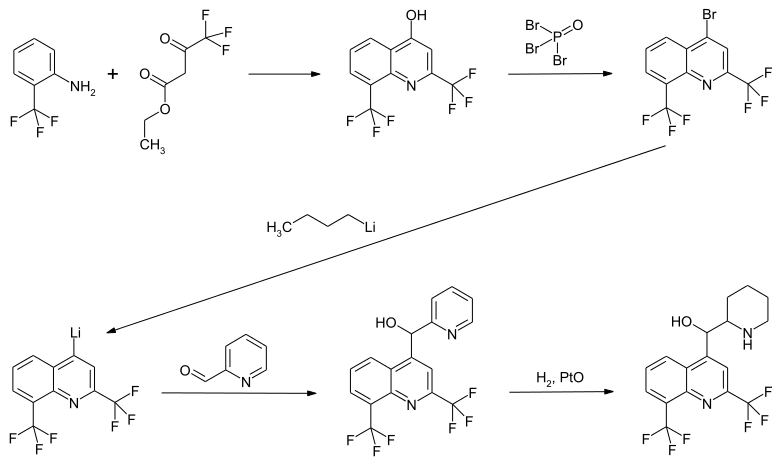
synthesis
Several syntheses for mefloquine are described in the literature. A process patented by Roche in 1978 describes a five-step synthesis sequence. In the first step, the reaction of 2-trifluoromethylaniline with ethyl trifluoroacetoacetate creates the basic quinoline structure. After substitution of the OH function by bromine using phosphorus oxybromide, lithiation using n -butyllithium and reaction with pyridine-2-carbaldehyde , the pyridyl precursor of the active ingredient is obtained. The target compound is then prepared by hydrogenating the pyridinyl function in the presence of platinum (II) oxide . In this synthesis, a racemate of the two enantiomeric erythro stereoisomers is obtained. Alternative synthetic routes also lead to the pyridinyl precursor, with the hydrogenation in turn forming the last synthetic step.
The synthetic routes for the selective preparation of the four possible stereoisomers are shown in the literature.
Chirality and structure-activity relationship
Mefloquine is a chiral molecule with two stereogenic centers and therefore has four different stereoisomers. Plasma concentrations of the (-) - enantiomers are significantly higher than those of the (+) - enantiomers and the pharmacokinetics of the two enantiomers are significantly different.
It has been suggested that the (+) - enantiomers are primarily effective in treating malaria, while the (-) - enantiomers are particularly effective in binding to the adenosine receptors of the CNS , which could explain some of the psychotropic side effects. A randomized double-blind study from 2010, on the other hand, could not determine any significant advantages of (+) - mefloquine compared to mefloquine racemate with regard to tolerability.
pharmacology
dosage
For an adult, a dose of 250 mg per week is recommended for prophylaxis (beginning two to three weeks before the expected exposure, ending four weeks afterwards). The therapeutic dosage is 750 mg mefloquine base at the start of therapy, six hours afterwards another 500 mg, twelve hours after another 250 mg. The substance is only excreted very slowly (see under side effects).
Side effects
The main side effects of mefloquine are neurological and psychiatric symptoms. These include u. a. Sleep disorders, nightmares / unusual dreams, dizziness, coordination and balance disorders , tinnitus , confusion, anxiety , depression , psychosis , hallucinations and convulsions. Should there be psychological changes (insomnia, nightmares / unusual dreams, acute anxiety states, depression, confusion, restlessness) during prophylactic use, these are to be regarded as " prodromal for more serious events" according to the manufacturer . In this case, the drug must be discontinued immediately and replaced with another antimalarial agent.
Mefloquine can also cause problems in the digestive tract such as nausea, diarrhea and vomiting. In addition, there are dermatological side effects (occasionally severe cases) and eye diseases.
Elimination takes a very long time, two to three weeks is the plasma half-life . Unwanted side effects can occur at any point in time and even after the drug is discontinued. In some cases, neuropsychiatric symptoms can last for months or years, or be permanent.
Contraindications and interactions
Mefloquine should not be taken if you are hypersensitive to quinine-like substances, nor should it be used if you have epilepsy or previous mental illnesses. The minimum weight of the user should be 5 kg. Particular caution must be exercised with kidney or liver diseases, cardiac arrhythmias and the intake of cardiovascular medication. During pregnancy should mefloquine by the manufacturer only in strict compliance with the indications are ingested. Basically, pregnant women or women who want to become pregnant are not advised before traveling to malaria areas.
Mefloquine must not be taken at the same time as Hypericum extracts ( St. John's wort extract ), as this can result in a loss of effectiveness of mefloquine. This loss of effectiveness was also found with the simultaneous use of the antibiotic rifampicin , which is why the simultaneous use of rifampicin and mefloquine should only be used in emergencies.
Due to the similarity of symptoms, there is a risk of confusing neurological and psychiatric side effects with the consequences of post-traumatic stress disorder or traumatic brain injury . The use of the drug by soldiers is therefore viewed critically.
Since September 2013, the manufacturer has also been listing contraindications for severe liver dysfunction and the occurrence of so-called blackwater fever. Blackwater fever (now known as hemoglobinuria ) is a serious complication of malaria in which the urine turns dark due to massive hemolysis.
Patient pass
In Germany, patients have been informed about possible side effects, interactions and contraindications of mefloquine since September 2013 by means of a so-called patient pass. The ID card should be kept permanently with you during the time it is taken and shown to every attending physician. The need to consult a doctor when neuropsychiatric symptoms appear, in order to immediately discontinue the medication and switch to another active ingredient (see also: Doxycycline , Atovaquone-Proguanil ) is expressly emphasized .
The German patient passport follows the example of the medication guide introduced in the USA in 2003 and last updated in July 2013. In July 2013, patient passports for mefloquine were also introduced in other EU countries.
Since October 6, 2014, the patient passport has been enclosed with every pack directly dispensed by Roche Pharma AG in Germany.
Sales figures
In Germany, the sales of mefloquine products have been falling sharply for several years. While in 2002 around 175,000 packs were sold through public pharmacies (original and imported), in 2012 the figure was around 37,000. In November 2015, the information service Apotheke Adhoc reported. In Germany, mefloquine is currently only "of subordinate importance in the prevention" of malaria. 88% of the antimalarial drugs sold in Germany in the previous twelve months were based on the active ingredient combination atovaquone-proguanil .
controversy
The use of mefloquine for the prophylaxis of malaria infections has been controversial since the drug was introduced. While the advantages lie on the one hand in the relatively good protection against infection with only weekly consumption, on the other hand there are repeated reports of neurological and psychiatric side effects. Studies that originally led to approval and that apparently showed good tolerability of the drug are characterized by methodological weaknesses ( e.g. lack of an adequate control group in a randomized, controlled study , non- representative patient collectives such as soldiers or prison inmates, problematic definitions of severe side effects). Randomized double-blind studies on average travelers with 976 and 623 participants respectively show neuropsychiatric side effects of varying severity in 29–37%. These values are statistically significantly higher than in the control groups.
In politics, the subject of mefloquine side effects has been raised repeatedly. In addition to the effects of the drug on civilians, its use by military personnel has repeatedly been criticized internationally. The then US Congressman Bart Stupak and Senator Christopher John Dodd demanded independent research in 2002 that should be subject to civil structures. In January 2005, Senator Dianne Feinstein (Democrats) wrote to then US Secretary of Defense Donald Rumsfeld to clarify a possible connection between the use of mefloquine and brainstem damage among US soldiers.
A study published in February 2006 with animal experiments on young rats showed a dose-dependent effect on the behavior of the animals up to the degeneration of nerve nuclei in the brain stem, whereby a threshold dose could be determined.
Furthermore, the news agencies United Press International and Associated Press reported in the years 2002-2005 on an accumulation of suicidal, paranoid and aggressive symptoms among Australian and US soldiers after taking mefloquine, so that this topic also gained prominence in the international media led to uncertainty.
At the general assembly in 2007, the then Chairman of the Board of Directors of Hoffmann La Roche AG, Franz B. Humer , partially distanced himself from mefloquine for malaria prevention: “In the past, LARIAM was the most important drug for combating malaria. As a result of the advancement of science, there are now more effective anti-malarial drugs that are better in terms of side effects and are mainly used. ”In certain cases, however, the drug is still used. The company stands by its responsibility.
In a memorandum dated February 2, 2009, the US Army Doctor General Eric Schoomaker initially downgraded mefloquine to a second-line drug for malaria prevention in soldiers. The drug is now a third-choice drug there and is only to be used prophylactically for US Army personnel with a contraindication to doxycycline and atovaquone-proguanil. In the USA, Roche discontinued the production and sale of mefloquine ( Lariam ) in summer 2009 . The drug is still available there as a generic . In other countries there was initially no withdrawal from the market.
Following the March 11, 2012 Kandahar massacre , of which United States Army Staff Sergeant Robert Bales was charged, media reports and the defendant's lawyers questioned whether the use of mefloquine had any effect on Bale's mental health at the time of the crime. In Germany, the use of the drug by soldiers of the Bundeswehr subsequently caused media coverage and political discussions.
In the summer of 2012, the US Food and Drug Administration (FDA) announced that it was reviewing mefloquine for possible severe balance disorders. In Germany, the drug received new specialist information in August of the same year, which emphasized the potential of neuropsychiatric disruptive effects much more strongly than before.
The TV magazine "Prime Time" of the Irish broadcaster RTÉ reported in May 2013 in a lengthy documentary about the use of Lariam (mefloquine) in the Irish army. The magazine's report focused on the relationship between use of the drug and cases of suicide and suicidality among Irish soldiers. Journalist Rita O'Reilly and producer Tara Peterman received the Irish Medical Journalist of the Year Award in November 2013 for their documentary.
In July 2013, the US Food and Drug Administration finally published new warnings on vestibular and psychiatric side effects . The updated warnings emphasize the possibility of very long and persistent neuropsychiatric symptoms. Attention is also drawn to the need to closely monitor children taking the drug, as adverse effects can be difficult to identify in this group of patients. A black box warning has also been introduced to highlight the severity and importance of the side effects .
Extended information on the adverse effects of mefloquine was also published in numerous countries of the European Union in the summer of the same year. In Germany, in September 2013, a Rote-Hand-Brief ( Rote-Hand-Brief) , a guide aimed at medical professionals, a checklist for prescribing and a patient passport were published in this context .
In response to the new warnings, the German Armed Forces downgraded mefloquine to a third-line drug for malaria prevention in the same month . According to the Parliamentary State Secretary Ralf Brauksiepe , it can be assumed that the drug has since been “not prescribed at all or only in very small amounts”. Exact figures are not available: "A determination of the number of prescriptions for malaria chemoprophylaxis with Lariam since October 2013 is not possible due to the individual, not digitally recorded prescription."
In the meantime, orders were given in the US Army to cease using the drug in elite units to prevent malaria. In Great Britain, the former chief of the British Army General Staff, Richard Dannatt , called for a ban on mefloquine for British forces as well. Concerns about its use were also expressed by the former chief of the British Defense Staff Charles Guthrie , the former commander of the 7th Armored Division Patrick Cordingley, the former commander of the British forces in Afghanistan, Richard Kemp, the former commander of the 3rd command brigade in the Falklands War, Julian Thompson, former Secretary of State for the United Kingdom Armed Forces Nick Harvey and British MPs Johnny Mercer and Douglas Chapman.
On October 24, 2013, the ARD television magazine Kontraste reported on possible deficiencies in the control system of the authorities responsible for drug safety. In the report of the magazine was u. a. criticized the late change in the technical information regarding the risk of suicide after taking Lariam ® (mefloquine). The Federal Institute for Drugs and Medical Devices (BfArM) looked due to the massive criticism compelled to issue a press release on October 25, 2013, the u. a. Questions about Lariam ® (mefloquine) answered and should contain further, detailed information about the safety measures implemented by the BfArM with regard to the pharmacovigilance of the drug.
The German Society for Tropical Medicine and International Health e. V. (DTG) deleted mefloquine as the drug of choice for the treatment of uncomplicated Malaria Tropica from its guidelines in November 2013. Since then, it has only been considered useful in individual cases. The reasons given are the new warnings of potentially serious side effects and the newly added contraindications.
At first, the DTG's position regarding the continued use of mefloquine for chemoprophylaxis remained unclear. In a statement dated October 22, 2013, the specialist society emphasized that the drug continues to play an important role in the prevention of malaria in certain cases. A DTG members' circular published towards the end of the same year mentioned discussions about possible tightening of the indications for the drug. According to this, mefloquine is an adequate chemoprophylaxis for malaria, especially for pregnant women. In a review published in January 2014, Gerd-Dieter Burchard, associate member of the Drugs Commission of the German medical profession , recommends co-author of the recommendations on malaria prevention of the German Society for Tropical Medicine and International Health. V. , at that time chairman of the DTG and then chairman of the guideline committee of the professional association, only to use mefloquine as a preventive measure "in exceptional cases".
On January 29, 2014, Roche in Switzerland, in consultation with the licensing authority Swissmedic, published a warning of possible eye diseases with mefloquine. According to the manufacturer, this includes u. a. Cataract , retinal abnormalities, and optic neuropathy that manifest as poor eyesight and blurred vision. These side effects can occur while taking or delayed. In addition, attention is drawn to cases with a very slow healing time and reports of “permanent secondary diseases”. If such side effects occur, the attending physician should be consulted in order to possibly discontinue the drug in consultation with him. Similar warnings had previously been published in other countries. In Germany, the Rote-Hand-Brief of September 2013 and the revision of the specialist information published in January 2014 emphasize: "Every patient who has visual disturbances should be referred to a doctor, as certain disorders (such as retinal diseases or optic neuropathy) require the May require treatment with mefloquine. "
For the Swiss market, the manufacturer Roche withdrew its mefloquine product Lariam on February 14, 2014. The drug is still approved for export there. Mefloquine is still on the market as a generic in Switzerland.
In Germany, the Federal Institute for Drugs and Medical Devices ordered the inclusion of persistent neuropsychiatric side effects in the product information of drugs containing mefloquine on February 27, 2014. It followed the new PRAC recommendation of the European Medicines Agency (EMA). This sees sufficient evidence for a causal connection between the intake of mefloquine and long-lasting and persistent neuropsychiatric disorders: "There is enough evidence from the presented drug safety reports, the submitted literature report and the FDA assessment report supporting a causal relationship between mefloquine and the occurrence of long lasting and even persistent neuropsychiatric side effects. "In this context, the strong suspicion is expressed that the drug can cause permanent brain damage even in prophylactic doses:" In consideration of this and the increase of case reports with long lasting side effects, there is a strong suspicion that mefloquine can cause different kind of permanent brain damage, even under plasma concentration achieved in malaria prophylaxis. "In the revision of the German technical information published in May of the same year, the manufacturer informed:" Side effects can occur even after stopping mefloquine. In a small number of patients it has been reported that neuropsychiatric side effects (e.g. depression, dizziness or vertigo, and balance disorders) can persist for months or longer even after discontinuing mefloquine. "
On June 1, 2014, the German Society for Tropical Medicine and International Hygiene e. V. new recommendations for malaria prophylaxis. Since then, mefloquine has been listed in third place behind atovaquone-proguanil and doxycycline as a drug that should only be used preventively if there is a “justified medical indication”. The observance of the special warning notices is emphasized. Regarding possible groups of people for whom the drug could be considered, it was initially said: "If the contraindications and warnings are observed, mefloquine still has an important role in malaria prophylaxis for pregnant women, children, migrants and long-term travelers as well as people who use the drug have repeatedly tolerated well. For prophylaxis, it is still an inexpensive alternative. ”In previous years, DTG listed mefloquine at the top of the recommended prophylactic drugs.
In October 2015, the manufacturer Roche announced that it would take Lariam off the market in Ireland on July 31, 2016. The company emphasized that the withdrawal was not related to ongoing legal disputes. On October 13 of the same year a settlement was reached between the manufacturer and an Irish plaintiff.
The House of Commons Defense Committee announced on October 13, 2015 an investigation into the use of mefloquine in the British Army. The interviewing of witnesses began on November 10th of the same year. In its final report published on May 24, 2016, the committee emphasized that in future mefloquine should only be used as a last resort under strict safety precautions. The previous conduct of the British Ministry of Defense was sharply criticized in this regard.
On February 24, 2016 the Deutsche Apotheker Zeitung (DAZ) published the announcement that the manufacturer Roche waived further approval of Lariam ® in Germany. In Denmark, Roche AG also waived the approval of Lariam in the same month .
In its updated recommendations from May 2016, the German Society for Tropical Medicine and International Health e. V., Mefloquine could continue to be prescribed under certain circumstances after the market withdrawal in Germany: “Since February 2016, the manufacturer has waived the approval of Lariam tablets (approval number 8634.00.00 - PZN: 04273048) in Germany. The marketability of the batches with the above-mentioned pharmaceutical central number remains due to the transition period (born in Section 31, Paragraph 4 AMG) until the shelf life expires. The company also announces that Lariam is available in many countries of the European Union and can therefore be obtained as a single import in accordance with Section 73 Paragraph 3 AMG. "This recommendation was questioned a few days later in the pharmaceutical-critical journal Arznei-Telegramm :" We do not see any noteworthy application niches, mainly because of the significant contraindications and the potential for severe and unpleasant adverse effects. "
On June 8, 2016, scientists at the Walter Reed Army Institute of Research (WRAIR) , which developed the active ingredient mefloquine in the 1970s, also published a scientific report on a military man with persistent neuropsychiatric symptoms after taking mefloquin and the difficulty of post-traumatic stress disorder (PTSD) to be distinguished from the consequences of mefloquine-induced toxicity (→ see section Contraindications and interactions ). The case is described of a 32-year-old man who received mefloquine at a dose of 250 mg / week for the prophylaxis of a malaria infection at the end of 2009 as a result of a military mission in East Africa. As early as two weeks after the first dose, neuropsychiatric symptoms such as vivid dreams, fears and balance disorders set in. However, he continued to take the drug for a period of four months. After that, in addition to the symptoms mentioned, other symptoms occurred that have persisted to this day and have severely restricted both the professional and private life of the man. A medication and psychotherapy for the treatment of complaints brought no improvement of the health condition. After application of a particular classification algorithm ( Naranjo - algorithm ) to the symptoms of a Mefloquininduzierte toxicity appears possible and probable. The conclusion from the case report states:
"This case documents the potential long-term and varied mefloquine-induced neuropsychiatric side effects, ranging from a central vestibulopathy to significant behavioral changes and sleep disorders. Especially pertinent to the military population, it demonstrates the difficulty in distinguishing from possible mefloquine-induced toxicity versus PTSD, and raises some questions regarding possible linkages between the two diagnoses. "
Translation:
“This case documents the possible long-term damage and various mefloquine-induced neuropsychiatric side effects, ranging from central vestibulopathy to significant changes in behavior and sleep disorders. In relation to members of the military in particular, it shows the difficulty in differentiating a possible mefloquine-induced toxicity from PTSD and raises some questions about possible links between the two diagnoses. "
In Canada, a public debate began in 2016 about the use of mefloquine in the local army. Here u. a. questioned the use of the drug during the United Nations operation in Somalia in 1992-93. The drug was not approved in Canada at the time. In addition, a potential connection between the killing of a Somali youth, known as the “Somalia Affair”, by members of the Canadian armed forces and the side effects of mefloquine was discussed.
In the Defense Committee of the German Bundestag , the Bundestag faction Bündnis 90 / Die Grünen submitted an application on September 28, 2016 to finally examine possible side effects of mefloquine in members of the Bundeswehr and to initiate research into toxicity. The Federal Ministry of Defense completely ended the use of mefloquine in the Bundeswehr on November 10 of the same year.
The parliamentary groups of the CDU / CSU and the SPD also submitted an application to the Defense Committee on February 2, 2018 to review mefloquine in the Bundeswehr. The focus should be on the extent to which the drug is used by members of the troops and possible long-term and permanent side effects. The Ministry of Defense is asked to submit a report by the end of 2018. In addition, military medical research on the potential toxicity of mefloquine is to be initiated. The motion was accepted with the votes of all parliamentary groups represented in the Bundestag with the exception of the AfD.
Shortly before, Roche sold the product rights for Lariam to the Greifswald-based pharmaceutical company Cheplapharm Arzneimittel . In New Zealand, as in numerous other countries before, the drug was withdrawn from the market in August 2018. In the same year, the Australian Senate started an investigation into test series with the active ingredients tafenoquine and mefloquine on members of the country's armed forces. This was preceded by years of ethical criticism of the studies in the media and in the medical community. The final report, published in December of the same year, found that the neuropsychiatric symptoms experienced by soldiers were authentic, with no clear explanation of the cause. Veterans were mostly disappointed about this in the press.
In June 2019, the German Society for Tropical Medicine and International Health eV published a heavily revised version of its recommendations on malaria prevention. In it, she relativizes the importance of the drug postulated in previous years, especially for migrants and children. The professional association now emphasizes: “If the contraindications and warnings are observed, mefloquine continues to play an important role in malaria prophylaxis and can still be considered as a cost-effective alternative. [...] For children and long-term travelers, simply taking it once a week is attractive. ”The recommendations appeared for the first time in 2019 in the specialist journal Flugmedizin, Tropical Medicine, Travel Medicine , in order to“ be independent of external sponsors ”according to the specialist association.
The withdrawal of approval of mefloquine parallel imports in Germany in May 2020 was not taken into account by the DTG in its recommendations from August of the same year. As before, it says: “There is, however, the possibility of obtaining parallel imported preparations approved in Germany. This means that there are still preparations containing mefloquine that can be used 'on label' according to the indication area. "
Suicidality
The US drug guide points to suicides after taking mefloquine and emphasizes that a causal connection to the intake of mefloquine has not yet been properly proven. A review from 2017 assumes a very low suicide rate based on the cases published in the specialist literature, but emphasizes the poor data basis. The German technical information also mentioned cases of suicide, suicide attempt, suicidal thoughts and self-endangering behavior. The reference in the 2010 version to an unproven causal relationship between suicidality and the use of the drug was deleted in the 2012 updated version. The Federal Institute for Drugs and Medical Devices justified these changes with “inconsistencies in the causality assessment of side effect reports carried out by Roche” and “a different assessment of the causal relationship” by the Federal Institute as part of an analysis of new assessment reports. In addition, the BfArM emphasizes that according to the European SmPC Guideline (Rev. 2, September 2009) no information about a questionable causality should be included in the section on side effects. In the patient passport introduced in Germany in 2013, the manufacturer wrote: "Lariam ® can cause serious mental problems in certain people, including suicide, suicidal thoughts and self- endangering behavior." An identical formulation is currently in the Austrian patient passport.
Pediatric use
The German Society for Tropical Medicine and International Hygiene e. V. (DTG) emphasized in the past the "important role" of mefloquine "in malaria prophylaxis in [...] children". Research by scientists who are close to the former mefloquine manufacturer Roche also supports the use of the agent in childhood. The assumption that mefloquine is better tolerated in small children than in adults is, however, doubted by other sources. In the United States, the package insert recommends vigilance in children who take mefloquine, as they can have difficulty identifying neuropsychiatric side effects. A case report from Germany published in 2018, which describes severe disruptive effects in a twelve-year-old girl, also raises the question of the exact age up to which mefloquine should be used in pediatrics.
In its revised version of the recommendations for malaria prevention from June 2019, the DTG relativizes its position on the use of mefloquine in children. It is true that the use of the agent in minors is "attractive" because it only needs to be taken once a week. Nonetheless, "[a] urchin [...] children [...] about the risk of neuropsychiatric side effects with the possibility of permanent damage, if necessary, should be comprehensively explained". In particular, “for older children and adolescents, for whom daily tablet intake is already possible”, “prophylaxis with atovaquone / proguanil should be preferred if there is no contraindication.” In contrast, the US package insert emphasizes the problem of neuropsychiatric side effects to recognize nonverbal children.
Application to migrants
The German Society for Tropical Medicine and International Hygiene e. V. (DTG) in recent years mefloquine as a prophylactic agent with "important [m] value". In a comment in the journal Travel Medicine and Infectious Disease with the participation of four authors of the DTG recommendations at the time and an author who worked part-time for Roche as a consultant, the drug was in accordance with this as a key prophylaxis ("key prophylaxis") for travelers who Visiting friends and relatives in malaria regions, named if they cannot afford the costs for more expensive alternatives or do not want to pay them ("those visiting friends and relatives (VFR) who cannot afford or will not pay for the more expensive chemoprophylaxis options") . In the new version of the recommendations of the DTG from 2019, the reference to the emphasized importance of mefloquine for migrants was deleted without replacement.
Use in the US prison camp at Guantanamo Bay
A 2010 report from the Seton University School of Law investigates whether the routine treatment of inmates with mefloquine in the Guantanamo Bay Naval Base detention center could have violated the rights of those concerned. All detainees in the camp were given a therapeutic dose of the drug. The treatment was carried out without diagnosis and consideration of contraindications. In the press, medical experts and politicians it was therefore speculated that side effects in the sense of pharmacological waterboarding were possibly consciously accepted.
Trivia and artistic receptions
- In his report "Kamboriam" from 1995, with an afterword by Adolf Muschg , the Swiss journalist Rolf Käppeli addresses the side effects of the malaria drug in partly fictionalized form.
- In the episode "Side Effects" of the US drama series Law & Order: Special Victims Unit from 2005, the investigators deal with a series of violent crimes committed under the influence of the fictional anti-malarial drug "Quinium".
- In his correspondence with the artist David Woodard , published in 2011, the writer Christian Kracht emphasizes that while he was in Somalia while under the influence of mefloquine he had deeply depressed thoughts and would rather run the risk of malaria than ever take the drug again.
- Ann Patchett's novel "River of Miracles" (original title: "State of Wonder") from 2011 describes the journey of a researcher into the Brazilian rainforest. During this time, the protagonist suffers from mefloquine-induced nightmares.
- The autobiography “The Answer to the Riddle Is Me” by US author David Stuart MacLean from 2014, subtitled “A memoir to Amnesia”, reports on massive memory loss under mefloquine.
- In the episode "Der Exorzismus der Anneliese E." of the German sitcom Das Institut - Oase des Scheiterns from 2019, one of the main characters suffers delusions and paranoia from taking a preventive antimalarial drug.
- The Belgian musician and fashion designer Stromae broke off a tour of Africa in 2015 because he suffered from anxiety from taking Lariam (mefloquine). Since then he has avoided public appearances. In a 2017 interview, he said he was still suffering from panic attacks.
Trade names
Monopreparations
Lariam (A), Mephaquin (CH), Mefloquin Acino (CH)
Web links
Individual evidence
- ↑ a b c data sheet MEFLOQUINE HYDROCHLORIDE CRS (PDF) at EDQM , accessed on February 5, 2009.
- ↑ a b entry on mefloquine. In: Römpp Online . Georg Thieme Verlag, accessed on March 10, 2014.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition. 2006, ISBN 0-911910-00-X , p. 1002.
- ↑ a b Data sheet Mefloquine hydrochloride from Sigma-Aldrich , accessed on April 9, 2011 ( PDF ).
- ↑ Obituary: Thomas R. Sweeney, chemical researcher . In: Washington Post. 4th July 2010.
- ↑ Rote-Hand-Brief on Lariam ®. (PDF) Roche Germany; published on the website of the Federal Institute for Drugs and Medical Devices (BfArM), September 2013.
- ↑ a b Lariam® specialist information ( Memento from February 21, 2014 in the Internet Archive ), Swiss Medicines Compendium , as of November 2013.
- ↑ How mefloquine works , EllVita health advice, as of 2013.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019.
- ↑ Guideline Diagnostics and Therapy of Malaria. ( Memento from January 13, 2014 in the Internet Archive ) German Society for Tropical Medicine and International Health e. V., October 2015. AWMF guidelines register 042/001.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019. Quotation: “It is possible to obtain preparations approved in Germany that have been imported in parallel. Thus, there are still preparations containing mefloquine that can be used "in-label" according to the indication area. "
- ↑ DeutschesArztPortal: Important drug information - general recalls DeutschesArztPortal. May 29, 2020.
- ↑ DeutschesArztPortal: Important drug information - general recalls DeutschesArztPortal. May 29, 2020.
- ↑ DeutschesArztPortal: Important drug information - general recalls DeutschesArztPortal. 4th June 2020.
- ↑ DeutschesArztPortal: Important drug information - general recalls DeutschesArztPortal. 23rd July 2020.
- ↑ DeutschesArztPortal: Important drug information - general recalls DeutschesArztPortal. 29th July 2020.
- ↑ Alexandra Negt: Lariam - revoke approval. Pharmacy Adhoc. 23rd July 2020.
- ↑ Alexandra Negt: Lariam "Pharma Gerke" has to go back. Pharmacy Adhoc. July 28, 2020.
- ^ Axel Kleemann, Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances. 4th edition. 2 volumes, Thieme-Verlag, Stuttgart 2000, ISBN 1-58890-031-2 ; online since 2003 with biannual additions and updates.
- ↑ ROTE LISTE 2008. Verlag Rote Liste Service, Frankfurt am Main, ISBN 978-3-939192-20-6 , p. 91.
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dietmar Reichert: Pharmaceutical Substances. 4th edition. 2 volumes, Thieme-Verlag, Stuttgart 2000, ISBN 1-58890-031-2 , pp. 1230-1231; online since 2003 with biannual additions and updates.
- ^ Patent DOS 2,806,909 (Roche, February 17, 1978).
- ^ RE Lutz, CJ powerlessness, AR Patel: Antimalarials. 7. Bis (trifluoromethyl) -. Alpha .- (2-piperidyl) -4-quinolinemethanol. In: J. Med. Chem. 14, 1971, pp. 926-928, doi: 10.1021 / jm00292a008 .
- ^ Patent DOS 2 940 443 (BASF, October 5, 1979).
- ↑ J. Ding, DG Hall: Concise Synthesis and Antimalarial Activity of All Four Mefloquine Stereoisomers Using a Highly Enantioselective Catalytic Borylative Alkenes Isomerization. In: Angew. Chem. 125, 2013, pp. 8227-8231, doi: 10.1002 / anie.201303931 .
- ↑ N. Schützenmeister, M. Müller, UM Reinscheid, C. Griesinger, A. Leonov: Trapped in Misbelief for Almost 40 Years: Selective Synthesis of the Four Stereoisomers of Mefloquine. In: Chem. Eur. J. 19, 2013, pp. 17584-17588, doi: 10.1002 / chem.201303403 .
- ↑ S. Baudry, YT Pham, B. Baune, S. Vidrequin, C. Crevoisier, F. Gimenez, R. Farinotti: Stereoselective passage of mefloquine through the blood-brain barrier in the rat. In: J Pharm Pharmacol. 49 (11), Nov 1997, pp. 1086-1090. PMID 9401943 .
- ↑ P. Schlagenhauf: Mefloquine for Malaria Chemoprophylaxis 1992–1998: A Review. In: J Travel Med. 6 (2), Jun 1999, pp. 122-133. PMID 10381965 . doi: 10.1111 / j.1708-8305.1999.tb00843.x
- ↑ S. Barraud de Lagerie, E. Comets, C. Gautrand, C. Fernandez, D. Auchere, E. Singlas, F. Mentre, F. Gimenez: Cerebral uptake of mefloquine enantiomers with and without the P-gp inhibitor elacridar ( GF1210918) in mice. In: Br J Pharmacol. 141 (7), Apr 2004, pp. 1214-1222. Epub 2004 Mar 15. PMID 15023856 .
- ^ M. Schmidt, H. Sun, P. Rogne, GK Scriba, C. Griesinger, LT Kuhn, UM Reinscheid: Determining the absolute configuration of (+) - mefloquine HCl, the side-effect-reducing enantiomer of the antimalaria drug Lariam . In: J Am Chem Soc. 134 (6), Feb 15, 2012, pp. 3080-3083. PMID 22148194 . doi: 10.1021 / ja209050k
- ↑ worldwide.espacenet.com
- ^ R. Tansley, J. Lotharius, A. Priestley, F. Bull, S. Duparc, J. Möhrle: A randomized, double-blind, placebo-controlled study to investigate the safety, tolerability, and pharmacokinetics of single enantiomer (+ ) -mefloquine compared with racemic mefloquine in healthy persons. In: The American journal of tropical medicine and hygiene. Volume 83, number 6, December 2010, pp. 1195–1201, doi: 10.4269 / ajtmh.2010.10-0228 . PMID 21118921 , PMC 2990031 (free full text).
- ↑ Malaria: Life-Saving Prophylaxis and Therapy. In: Pharmaceutical newspaper online.
- ↑ Guideline: Diagnosis and Therapy of Malaria. (PDF) German Society for Tropical Medicine and International Health (DTG) of 23 August 2011 (PDF; 271 kB)
- ↑ BfArM: Pharmacovigilance for Mefloquine ( Memento of October 22, 2013 in the Internet Archive )
- ↑ Lariam prescribing information , Roche Pharma AG, August, 2015.
- ↑ Pharmacovigilance Risk Assessment Committee (PRAC). (PDF) Minutes of the meeting on October 23-26, 2017, European Medicines Agency, p. 31.
- ↑ Lariam prescribing information (Austria), Roche Austria GmbH February 2018th
- ↑ FDA Drug Safety Communication: FDA approves label changes for antimalarial drug mefloquine hydrochloride due to risk of serious psychiatric and nerve side effects.
- ↑ Mefloquine-containing medicinal products: persistent neuropsychiatric side effects. ( Memento from August 28, 2014 in the Internet Archive ) Risk information, Federal Institute for Drugs and Medical Devices (BfArM), February 27, 2014.
- ↑ A. Ringqvist, P. Bech, B. Glenthøj, E. Petersen: Acute and long-term psychiatric side effects of mefloquine: A follow-up on Danish adverse event reports. In: Travel medicine and infectious disease. [electronic publication before printing] November 2014, doi: 10.1016 / j.tmaid.2014.10.021 , PMID 25435322 .
- ↑ technical information Lariam , Roche Pharma AG, August, 2015.
- ↑ P. Schlagenhauf, WA Blumentals, P. Suter, L. Regep, G. Vital-Durand, MT Schaerer, MS Boutros, HG Rhein, M. Adamcova: Pregnancy and fetal outcomes after exposure to mefloquine in the pre- and periconception period and during pregnancy. In: Clinical Infectious Diseases . Volume 54, number 11, June 2012, pp. E124 – e131, doi: 10.1093 / cid / cis215 . PMID 22495078 , PMC 3348951 (free full text).
- ^ RL Nevin: Limitations of postmarketing surveillance in the analysis of risk of pregnancy loss associated with maternal mefloquine exposure. In: Clin Infect Dis. 55 (8), Oct 2012, pp. 1167-1168; author reply 1168–1169. Epub 2012 Jul 3rd PMID 22761410 .
- ^ RL Nevin: Mefloquine gap junction blockade and risk of pregnancy loss. ( Memento from March 12, 2015 in the Internet Archive ) In: Biol Reprod. 87 (3), 21 Sep 2012, p. 65. Print 2012 Sep. PMID 22837476 .
- ↑ Lariam® patient information ( Memento from February 21, 2014 in the Internet Archive ), Swiss Medicines Compendium , as of October 2013.
- ^ R. Nevin: Mefloquine and posttraumatic stress disorder. ( Memento of March 4, 2016 in the Internet Archive ) In: EC Ritchie (Ed.): Textbook of Military Medicine. Forensic and Ethical Issues in Military Behavioral Health. Borden Institute, Washington, DC 2015, pp. 277-296.
- ^ R. Nevin: Rational Risk-Benefit Decision-Making in the Setting of Military Mefloquine Policy. In: J Parasitol Res. 2015, p. 260106. PMID 26579231 .
- ^ R. Nevin, EC Ritchie: The Mefloquine Intoxication Syndrome: A Significant Potential Confounder in the Diagnosis and Management of PTSD and Other Chronic Deployment-Related Neuropsychiatric Disorders. In: Posttraumatic Stress Disorder and Related Diseases in Combat Veterans. Springer, Berlin / Heidelberg 2015, pp. 257–278. doi : 10.1007 / 978-3-319-22985-0_19
- ^ AJ Magill, MA Forgione, JD Maguire, MM Fukuda: Special Considerations for US Military Deployments ( Memento June 10, 2015 in the Internet Archive ). In: Yellow Book 2014. Centers for Disease Control, Atlanta 2014.
- ^ AJ Magill: Special Considerations for US Military Deployments. In: Yellow Book 2016. Centers for Disease Control, Atlanta 2015.
- ↑ BfArM: Pharmacovigilance for Mefloquine ( Memento of October 22, 2013 in the Internet Archive ).
- ↑ Rote-Hand-Brief on Lariam ®. ( Memento from October 22, 2013 in the Internet Archive ) Roche Germany; published on the website of the Federal Institute for Drugs and Medical Devices (BfArM), September 2013.
- ^ FDA Creates Medication Guide for Lariam. ( Memento from April 1, 2013 on the Internet Archive ) US Food & Drug Administration press release, June 9, 2003.
- ↑ Agence nationale de sécurité du médicament et de produits de santé: LARIAM® (méfloquine): actualisation du profil de tolérance Information importante de Pharmacovigilance - Lettre aux professionnels de santé. ( Memento from August 13, 2013 in the web archive archive.today ) July 8, 2013.
- ↑ Direct Healthcare Professional Communication on Lariam (R) (mefloquine) for malaria chemoprophylaxis and the risk of neuropsychiatric adverse reactions. (PDF; 1.6 MB) Roche Ireland. July, 1st 2013.
- ↑ Cave Lariam®. In: Dtsch Arztebl. 112 (35-36), 2015, pp. A-1428
- ↑ Ed .: CNS-toxic mefloquine (Lariam) still to be justified as an anti-malarial agent? (PDF) In: Arznei-Telegram. Vol. 44, No. 8, 2013, p. 72.
- ↑ Patrick Hollstein: Generics hijack the malaria market. In: Apotheke Adhoc. 3rd November 2015.
- ↑ AM Croft, P. Garner: Mefloquine for preventing malaria in non-immune adult travelers. In: Cochrane Database Syst Rev. (4), 2000, Art. No. CD000138. PMID 11034675
- ^ AM Croft, DP Whitehouse: Prophylaxis against malaria. More studies of mefloquine prophylaxis must be done in tourists. In: BMJ . 318 (7191), Apr 24, 1999, pp. 1139-1140. PMC 1115531 (free full text).
- ↑ AM Croft: Healthy people need safe drugs, too. Lessons from Lariam and Halfan. ( MS Word ; 70 kB). Health Action International Europe Conference 2004.
- ↑ D. Overbosch, H. Schilthuis, U. Bienzle, RH Behrens, KC Kain, PD Clarke, S. Toovey, J. Knobloch, HD Nothdurft, D. Shaw, NS Roskell, JD Chulay; Malarone International Study Team: Atovaquone-proguanil versus mefloquine for malaria prophylaxis in nonimmune travelers: results from a randomized, double-blind study. ( Memento of October 16, 2013 in the Internet Archive ) (PDF; 1.2 MB) In: Clin Infect Dis . 33 (7), Oct 1, 2001, pp. 1015-1021.
- ↑ P. Schlagenhauf, A. Tschopp, R. Johnson, HD Nothdurft, B. Beck, E. Schwartz, M. Herold, B. Krebs, O. Veit, R. Allwinn, R. Steffen: Tolerability of malaria chemoprophylaxis in non -immune travelers to sub-Saharan Africa: multicentre, randomized, double blind, four arm study. In: BMJ. 327 (7423), Nov 8, 2003, p. 1078. PMC 261741 (free full text).
- ↑ Tickell-Painter M, Maayan N, Saunders R, Pace C, Sinclair D: Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database Syst Rev. 2017 Oct 30; 10: CD006491. PMID 29083100 . PMC 5686653 (free full text)
- ^ Jean Corston (Member of Parliament): Parliamentary question on the side effect profile of mefloquine. British House of Commons, March 1997.
- ↑ Senator Feinstein Urges CDC To Review Its Recommendations on Anti-Malarial Drug. Press release by US Senator Dianne Feinstein (Democrats). June 2004.
- ↑ Senator Feinstein Urges Pentagon to Step Up Efforts to Track Medical Damage Caused by Anti-Malarial Drug. Press release by US Senator Dianne Feinstein (Democrats). June 2004.
- ↑ Senator Feinstein Urges Secretary Powell to Reassess Lariam Use by State Department Employees. ( Memento of October 16, 2013 in the Internet Archive ) (PDF; 188 kB) Press release by US Senator Dianne Feinstein (Democrats). July 2004.
- ^ Democratic Policy Committee, p. 1042, the National Defense Authorization Act for Fiscal Year 2006. ( Memento of March 27, 2008 in the Internet Archive ) May 24, 2005.
- ↑ Feinstein Calls for Review of Anti-malarial Drug. Press release from US Senator Dianne Feinstein (Democratic Party). August 2011.
- ↑ M. Benjamin, D. Olmsted: Army Fort Bragg study faces scrutiny. ( Memento of July 29, 2007 in the web archive archive.today ) In: United Press International. November 8, 2002.
- ↑ Senator Feinstein Urges Rumsfeld to Complete Lariam Study. ( Memento of March 8, 2011 in the Internet Archive ) (PDF; 31 kB) Press release by US Senator Dianne Feinstein (Democrats). January 2005.
- ↑ G. Dow et al. a., Walter Reed Army Institute of Research: Mefloquine induces dose-related neurological effects in a rat model. In: Antimicrob Agents Chemother . 50 (3), Mar 2006, pp. 1045-1053. PMC 1426433 (free full text).
- ^ R. Burns: Army sending health experts to Fort Bragg to look for links to spousal killings. Associated Press, Aug 23, 2002.
- ↑ M. Benjamin, D. Olmsted: Navy coverup alleged on drug side effects. ( Memento of July 29, 2007 in the web archive archive.today ) In: United Press International. September 8, 2003.
- ↑ Report: Lariam Affects Aussie troops. In: United Press International. September 29, 2005.
- ↑ Minutes of the 89th Annual General Meeting of Shareholders of Roche Holding AG, Basel (PDF; 503 kB) on March 5, 2007, 10.30 a.m., in the Basel Exhibition Center, Basel.
- ↑ Eric Schoomaker. Department of the Army. Office of the Surgeon General: Updated guidance on Use of Mefloquine (Lariam®) for Malaria Prophylaxis. ( Memento of July 16, 2011 in the Internet Archive ) (PDF; 493 kB). February 2, 2009.
- ↑ Centers for Disease Control (CDC): Special Considerations for US Military Deployments. ( Memento from June 10, 2015 in the Internet Archive ) In: Yellow Book. Centers for Disease Control, Atlanta 2013.
- ↑ Jonathan Woodson: Guidance on Medications for Prophylaxis of Malaria. ( Memento of October 4, 2013 in the Internet Archive ) (PDF; 2.9 MB) Department of Defense, April 15, 2013.
- ↑ Associated Press: Army curbs prescriptions of anti-malaria drug . In: USA Today. November 19, 2011.
- ↑ Mark Benjamin: Robert Bales Charged: Military Scrambles To Limit Malaria Drug Just After Afghanistan Massacre. In: Huffington Post. March 25, 2012, accessed March 27, 2012 .
- ↑ Amy Goodman, Mark Benjamin: Pentagon Stays Silent on Whether Suspect in Afghan Massacre Took Controversial Anti-Malaria Drug. In: Democracy Now. March 27, 2012, accessed March 27, 2012 .
- ↑ Elspeth Cameron Ritchie: A Smoking Pillbox: Evidence that Sgt. Bales May Have Been on Lariam. In: Time Magazine. June 20, 2013.
- ↑ Hal Berton: Did malarial drug play role in Bales' Afghan murders? In: Seattle Times. 18th July 2013.
- ↑ Nina Shapiro: Why Robert Bales' Lawyer Kept Mum on Mefloquine. ( Memento from August 27, 2013 on the Internet Archive ) In: Seattle Weekly. August 26, 2013.
- ^ Adam Ashton: Army: Robert Bales' medical records to remain classified. In: The News Tribune. 3rd September 2014.
- ↑ Elizabeth McLaughin: Lawyers claim anti-malaria drug to blame in US soldier's Afghan massacre. ABC News, May 16, 2018.
- ↑ Patricia Kime: Ex-soldier who killed 16 Afghan civilians may seek clemency from president, lawyer says. The Daily World, June 20, 2018.
- ↑ Contrasts: Risk for soldiers - Bundeswehr relies on controversial malaria drug. ARD, April 12, 2012.
- ↑ Contrasts: Risky Malaria Prophylaxis - How the Bundeswehr downplayed the dangers. ARD, May 3, 2012.
- ↑ Anti- malarial drugs make soldiers uncontrollable. In: Frankfurter Rundschau. April 13, 2012.
- ↑ Small question from MPs Inge Höger, Christine Buchholz, Sevim Dagdelen, Annette Groth, Andrej Hunko, Harald Koch, Stefan Liebich, Niema Movassat, Kathrin Vogler, Harald Weinberg and the DIE LINKE parliamentary group: Use of the malaria drug Lariam (Mefloquin) in the Bundeswehr . (PDF) German Bundestag, 17th electoral term. Printed matter 17/9789. May 16, 2012 (PDF; 95 kB).
- ↑ Answer of the Federal Government to the small question of the MPs Inge Höger, Christine Buchholz, Sevim Dagdelen, other MPs and the parliamentary group DIE LINKE. (PDF; 252 kB) German Bundestag, 17th electoral term. Printed matter 17/10075. June 25, 2012.
- ↑ Omid Nouripour (Bündnis 90 / Die Grünen): Written question: Consequences of the Bundeswehr with regard to the malaria drug Lariam because of psychological side effects. German Bundestag, 17th electoral term. BT-Drs. 17/12239 (written questions).
- ^ Potential Signals of Serious Risks / New Safety Information Identified by the Adverse Event Reporting System (AERS) between April - June 2012. Food and Drug Administration (FDA), Atlanta 2012.
- ↑ Roche Pharma AG: Lariam SPC. August 2012, slightly modified in November 2012.
- ↑ RTÉ's Investigations Unit asks if the anti-malarial drug Lariam be linked to suicides among Irish Defense Forces soldiers. RTÉ television magazine Prime Time, May 2013.
- ↑ Six Irish Times journalists receive medical media awards. In: Irish Times. November 7, 2013.
- ↑ FDA Drug Safety Communication: FDA approves label changes for antimalarial drug mefloquine hydrochloride due to risk of serious psychiatric and nerve side effects.
- ↑ Petra Jungmayr: Neurological and psychiatric side effects: Black box for mefloquine. In: Deutsche Apothekerzeitung. August 11, 2013.
- ↑ Malaria: Vestibular and Psychiatric Aftermath of Mefloquine. In: Deutsches Ärzteblatt. July 30, 2013.
- ↑ Lariam: Summary of Product Characteristics ( Memento of 12 October 2013 Internet Archive ) Roche Ireland June, 2013.
- ↑ Agence nationale de sécurité du médicament et de produits de santé: LARIAM® (méfloquine): actualisation du profil de tolérance Information importante de Pharmacovigilance - Lettre aux professionnels de santé. July 8, 2013.
- ↑ Comunicazione diretta agli operatori sanitari su Lariam (meflochina) per la chemioprofilassi della malaria e rischio di reazioni avverse neuropsichiatriche. (PDF) L'Agenzia Italiana Del Farmaco, June 2013 (PDF; 59 kB)
- ↑ Lariam: Doprovodné texty. Roche Česká Republika, August 21, 2013.
- ↑ Package leaflet: Information for the user. Lariam 250 mg tablets. Roche Belgium, 8/2013.
- ↑ Rote-Hand-Brief on Lariam ®. ( Memento from October 22, 2013 in the Internet Archive ) Roche Germany; published on the website of the Federal Institute for Drugs and Medical Devices (BfArM), September 2013.
- ↑ Kathrin Vogler: Written inquiry: Use of the malaria drug Lariam ® in Bundeswehr soldiers. (PDF) German Bundestag. 17th legislative term. Printed matter 17/14813. October 4, 2013, pp. 35–36.
- ↑ Inge Hoeger: Written inquiry: Use of the malaria drug Lariam ® in Bundeswehr soldiers. (PDF) German Bundestag. 18th legislative term. Printed matter 18/36. November 8, 2013, pp. 39-41.
- ↑ In focus: health protection in action. Interview with Chief Medical Officer Salvatore Schmidt. Published on the website of the Bundeswehr Medical Service, October 23, 2013.
- ↑ Thomas Wiegold : After the alarm: (Almost) an end to Lariam in the Bundeswehr. In: eyes straight ahead! November 7, 2013.
- ↑ Ralf Brauksiepe: Answer to written request from Brugger, Agnieszka: German soldiers with the prescribed drug Lariam for malaria prophylaxis. (PDF) German Bundestag. 18th legislative term. Printed matter 18/8191. April 22, 2016, pp. 26-27.
- ↑ Paula Jelineck: Green Berets, other elite Army forces ordered to stop taking anti-malarial drug mefloquine. ( Memento from September 29, 2013 in the web archive archive.today ) In: Washington Post. 19th September 2013.
- ↑ Jonathan Owen: The Lariam scandal: Former head of army calls for ban on malaria drug. In: The Independent. September 27, 2013.
- ↑ Jonathan Owen: British military chiefs demand review into continued use of Lariam. In: The Independent. October 7, 2013.
- ↑ Marco Giannangeli: War hero issues alert over controversial antimalarial drug. In: Sunday Express. 20th July 2014.
- ↑ Jonathan Owen: Soldiers still suffering serious mental illness linked to controversial anti-malarial drug Lariam. In: The Independent. November 28, 2014.
- ↑ Sima Kotecha: Call for Army to stop using malaria drug mefloquine. In: BBC News. 17th August 2015.
- ^ SNP MP calls for answers on anti malarial drug use. ( September 26, 2015 memento on the Internet Archive ) Scottish National Party press release , September 13, 2015.
- ↑ Jonathan Owen: Lariam: Select committee to consider inquiry over MoD's use of anti-malarial drug. In: The Independent. 15th September 2015.
- ^ A. O'Dowd: MPs may hold inquiry into safety of using antimalarial mefloquine in military. In: BMJ. 351, Sep 11, 2015, p. H4868. PMID 26361783 .
- ↑ Life-threatening risks - drug control authorities fail. ARD television magazine Kontraste, October 24, 2013.
- ↑ Contrasts article on Flupirtin and Lariam: BfArM offers further information on the Internet. ( Memento from February 22, 2014 in the Internet Archive ) BfArM press release 12/13, October 25, 2013.
- ↑ Guideline Diagnostics and Therapy of Malaria. ( Memento from January 13, 2014 in the Internet Archive ) German Society for Tropical Medicine and International Health e. V., November 2013. AWMF guidelines register 042/001.
- ↑ Recommendations for the prevention of malaria. German Society for Tropical Medicine and International Health V., April 2013. Amendment from October 22, 2013.
- ↑ Travel medicine Malaria prophylaxis for pregnant women in pharmische-zeitung.de edition 24/2010.
- ↑ Malaria recommendations for prevention and emergency self-treatment ( Memento from March 4, 2011 in the Internet Archive ) Health Service Regional Doctor Yaounde.
- ^ GD Burchard: Malaria. In: Internist (Berl). Jan 9, 2014, pp. 1-12, PMID 24399475 .
- ↑ Roche Pharma (Switzerland) AG: Increased risk of eye diseases in connection with the use of Lariam ® (mefloquine). ( Memento of February 1, 2014 in the Internet Archive ) Published on the Swissmedic website. January 29, 2014.
- ^ Mefloquine and visual disturbances. (PDF) Prescriber Update 2013; 34 (4) December. New Zealand Medicines and Medical Devices Safety Authority, p. 48.
- ↑ Mefloquine hydrochloride (Lariam): Safety advisory - potential for visual disturbances. Australian Government, Department of Health. October 11, 2013.
- ↑ Important new safety information for Lariam (mefloquine) regarding visual disturbances including optic neuropathy. Drug Office. Department of Health. Hong Kong. July 16, 2013.
- ↑ Rote-Hand-Brief on Lariam ®. ( Memento from October 22, 2013 in the Internet Archive ) Roche Germany; published on the website of the Federal Institute for Drugs and Medical Devices (BfArM), September 2013.
- ↑ Roche Pharma AG: Lariam SPC. January 2014.
- ↑ Swissmedic Journal 2/2014 ( Memento from June 6, 2014 in the Internet Archive ). Official publication organ of Swissmedic, Schweizerisches Heilmittelinstitut, Bern, p. 134.
- ↑ Mefloquine-containing medicinal products: persistent neuropsychiatric side effects. ( Memento of May 8, 2014 in the Internet Archive ) Federal Institute for Drugs and Medical Devices , February 27, 2014.
- ↑ db: Malaria: Mefloquine has long-term effects on the psyche. In: Pharmaceutical newspaper. February 28, 2014.
- ↑ Updated PRAC rapporteur assessment report on the signal of permanent neurologic (vestibular) disorders with mefloquine. (PDF) European Medicines Agency, January 31, 2014.
- ↑ Lariam prescribing information , Roche Pharma AG May, 2014.
- ↑ Recommendations for the prevention of malaria. German Society for Tropical Medicine and International Health V., May 2014. The wording corresponds to the addition of the recommendations from October of the previous year. See recommendations for malaria prevention. German Society for Tropical Medicine and International Health V., April 2013. Amendment from October 22, 2013.
- ↑ Recommendations for the prevention of malaria. (PDF) German Society for Tropical Medicine and International Health e. V., May 2016.
- ↑ Recommendations for the prevention of malaria . German Society for Tropical Medicine and International Health V., March 2010.
- ↑ Recommendations for the prevention of malaria. ( Memento from December 26, 2014 in the Internet Archive ) German Society for Tropical Medicine and International Health e. V., April 2011.
- ↑ Recommendations for the prevention of malaria . German Society for Tropical Medicine and International Health V., April 2013.
- ↑ Shane Phelan: Anti-malarial drug to be withdrawn from Irish market. In: Irish Independent. October 14, 2015.
- ^ Mary Carolan: Action over alleged effects of anti-malarial Lariam settled. In: The Irish Times. October 13, 2015.
- ↑ Mark Tighe: First pay-out over drug blamed for soldiers' suicides. The Times. October 14, 2015.
- ↑ Jonathan Owen: Lariam: Inquiry to be held into MoD's use of anti-malaria drug on British soldiers amid health concerns. In: The Independent. October 14, 2015.
- ↑ Deborah Haynes: Anti-malaria drug to face MPs' scrutiny. In: The Times. October 14, 2015.
- ↑ Lariam inquiry. Information on the website of the United Kingdom Parliament, UK Defense Committee.
- ↑ Larisa Brown: Malaria pill for forces is a risk, admit drug chiefs: Controversial treatment found to increase risk of depression, anxiety and psychosis. In: Daily Mail. November 11, 2015.
- ↑ Jonathan Owen: Lariam: Medical experts condemn MoD for giving soldiers anti-malarial drug with psychiatric side effects. In: The Independent. December 8, 2015.
- ↑ Sam Blackledge: Ministry of Defense admits breaching guidelines by giving Lariam drug to troops. ( Memento of January 29, 2016 in the Internet Archive ) In: Plymouth Herald. January 12, 2016.
- ↑ Bern Farmer: More than 1 in 20 troop deaths happen in training. In: The Telegraph. January 12, 2016.
- ↑ Sima Kotecha: Lariam should be drug of last resort for troops, MPs say. In: BBC News. May 24, 2016.
- ↑ Julia Borsch: Lariam antimalarial agent will only be available as an import in future. In: Deutsche Apotheker Zeitung. (DAZ), February 24, 2016.
- ↑ The silent departure of the malaria drug mefloquine (LARIAM). In: Medicinal Telegram. 47, 2016, pp. 31–32.
- ↑ Lariam® is no longer marketed in Denmark. In: EPI-News , 7/8, 2016, Statens Serum Institut.
- ↑ Recommendations for the prevention of malaria. (PDF) German Society for Tropical Medicine and International Health e. V., May 2016.
- ↑ Malaria prophylaxis after the mefloquine (Lariam) withdrawal. In: Medicinal Telegram. Vol. 47, No. 6, 2016, pp. 57–59.
- ↑ Jeffrey Livezey, Thomas Oliver, Louis Cantilena: Prolonged Neuropsychiatric Symptoms in a Military Service Member Exposed to Mefloquine. In: Drug Safety - Case Reports. 3, 2016, doi: 10.1007 / s40800-016-0030-z .
- ^ Sheila Pratt: Risky anti-malaria drug given to thousands of Canadian veterans. The Star, May 25, 2016.
- ↑ Chris Doucette: Former soldier suffers after taking anti-malaria drug. Toronto Star, December 18, 2016.
- ↑ Chris Doucette: Veterans, families want answers over Forces' use of Mefloquine. Toronto Sun, Jan 23, 2017
- ^ Cullen Crozier: APTN Investigates: Bad Medicine. APTN National News, April 7, 2017.
- ↑ Agnieszka Brugger , Tobias Lindner , Doris Wagner : Motion of the Bündnis 90 / Die Grünen parliamentary group in the Defense Committee of the German Bundestag on the draft of Section 14, Chapter 1403, Title Group 01. (PDF) German Bundestag. Defense Committee. Reject Document 18 (12) 786. Published on the website of Agnieszka Brugger (Bündnis 90 / Die Grünen, Member of the Bundestag).
- ^ Sheila Pratt: Germany bans drug linked to brain damage, ramps up pressure on Canada. iPolitics, December 9, 2016.
- ^ Agnieszka Brugger: End for the controversial Lariam drug in the Bundeswehr. Press release, December 15, 2016. Published on Agnieszka Brugger's website (Bündnis 90 / Die Grünen, Member of the Bundestag).
- ↑ Rachel Riley: Vets back ban on anti-malaria drug. Townsville Bulletin, December 11, 2016.
- ↑ Addam Higgins: Irish Defense Forces blasted for continuing to give troops 'dated' Lariam pills that leave some soldiers with suicidal thoughts. The Irish Sun, December 16, 2016.
- ^ Motion from the CDU / CSU and SPD parliamentary groups in the Defense Committee of the German Bundestag on the federal government's draft law. Printed matter 19/1700, 19/1701. Draft of a law on the adoption of the federal budget for the budget year 2018 (Budget Law 2018). (PDF) German Bundestag. Printed matter 19/2426.
- ↑ Cheplapharm remains on course for growth and complements its portfolio with four further products. Press release from Cheplapharm, January 26, 2018.
- ↑ Discontinuation of Lariam® (Mefloquine) 250mg × 8 tablets. (PDF) Roche New Zealand, press release, August 15, 2018.
- ↑ Use of the Quinoline anti-malarial drugs Mefloquine and Tafenoquine in the Australian Defense Force. Australian Parliament website, Senate Foreign Affairs Defense and Trade References Committee, June 2018.
- ↑ Michael Atkin: It's destroyed my life: Hopes inquiry will back veterans claims anti-malaria drug caused illness. ABC News. July 31, 2018.
- ^ Paul Cleary: Drug trial a test of ethics. The Australian, 9/11/2015.
- ↑ Mandie Sami: Defense Force accused of 'massive cover-up' over anti-malarial drug. ABC News, December 4th, 2015.
- ^ Jesse Dorsett: Former soldiers, families face military officials in Townsville over anti-malaria drug side effects. ABC News, 3/14/2016.
- ↑ Henry Belot: Therapeutic Goods Administration warned military doctors before using experimental drug on soldiers. Sydney Morning Herald, April 29, 2016.
- ^ S. McCarthy: Malaria Prevention, Mefloquine Neurotoxicity, Neuropsychiatric Illness and Risk-Benefit Analysis in the Australian Defense Force. (PDF) In: J Parasitol Res. 2015, p. 287651.
- ↑ Use of the Quinoline anti-malarial drugs Mefloquine and Tafenoquine in the Australian Defense Force. Executive summary. Internet presence of the Australian Parliament, Senate Foreign Affairs Defense and Trade References Committee, December 2018. Quote: “The committee needs to state that it is not comprised of medical professionals or health experts and so cannot make any findings or rulings in relation to the medical causes for health issues. However, it notes that the weight of prevailing medical evidence provided to the committee in response to these claims is that long term problems as a result of taking mefloquine are rare and there is no compelling evidence that tafenoquine causes long term effects. To be clear, there has always been recognition by Defense that mefloquine, like any drug, has side effects and this has been taken into consideration in the development of its health policy. "
- ↑ Rachel Eddie: 'Disheartening': Veterans 'let down' by inquiry into anti-malarial drug trials. The New Daily, December 5, 2018.
- ↑ a b Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., August 2020.
- ↑ Tickell-Painter M, Saunders R, Maayan N, Lutje V, Mateo-Urdiales A, Garner P: Deaths and parasuicides associated with mefloquine chemoprophylaxis: A systematic review. Travel Med Infect Dis. 2017 Nov - Dec; 20: 5-14. Epub 2017 Nov 2. doi: 10.1016 / j.tmaid.2017.10.011 . PMID 29107173
- ↑ Roche Pharma AG: Lariam SPC. December 2010.
- ↑ Roche Pharma AG: Lariam SPC. August 2012, slightly modified in November 2012.
- ↑ Information from the Federal Institute for Drugs and Medical Devices on Flupirtine and Lariam. (PDF) Federal Institute for Drugs and Medical Devices, October 25, 2013.
- ↑ SmPC Guideline Rev.2, September 2009. (PDF) EudraLex of the European Commission
- ↑ Red Hand Letter. (PDF; 2.2 MB).
- ↑ Austria: Official training material for medical professionals for Lariam® 250 mg. Patient pass. Cheplapharm 2018.
- ↑ Recommendations for the prevention of malaria. German Society for Tropical Medicine and International Health V., May 2018.
- ↑ P Schlagenhauf, M Adamcova, L Regep, MT Schaerer, S Bansod, HG Rhein: Use of mefloquine in children - a review of dosage, pharmacokinetics and tolerability data. In: Malar J. , 2011 Oct 7; 10, p. 292, doi: 10.1186 / 1475-2875-10-292 , PMC 3215676 (free full text), PMID 21981927
- ^ Nevin R: Biased Measurement of Neuropsychiatric Adverse Effects of Pediatric Mefloquine Treatment. In: Pediatr Infect Dis J. , Jan 2011 31; 1, p. 102. PMID 22217970 .
- ↑ FDA Drug Safety Communication: FDA approves label changes for antimalarial drug mefloquine hydrochloride due to risk of serious psychiatric and nerve side effects.
- ↑ R Rieke: Mefloquin: The dead are longer harmful. (PDF) In: Flug u Reisemed , 2018; 25, pp. 153-156.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019.
- ↑ FDA Drug Safety Communication: FDA approves label changes for antimalarial drug mefloquine hydrochloride due to risk of serious psychiatric and nerve side effects.
- ↑ Recommendations for the prevention of malaria. German Society for Tropical Medicine and International Health V., May 2018.
- ↑ Schlagenhauf P, Hatz C, Behrens R, Visser L, Funk M, Holzer B, Beck B, Bourquin C, Etter H, Furrer H, Genton B, Landry P, Chappuis F, Loutan L, Stössel U, Jeschko E, Rossanese A, Nothdurft HD: Mefloquine at the crossroads? Implications for malaria chemoprophylaxis in Europe. Travel Med Infect Dis. 2015 Mar-Apr; 13 (2): 192-6. PMID 25825015
- ↑ Recommendations for the prevention of malaria. German Society for Tropical Medicine and International Health V., April 2013. Amendment from October 22, 2013.
- ↑ Mark Tighe: Army's Lariam expert funded by drug maker. The Sunday Times, October 27, 2013.
- ↑ Malaria prophylaxis - recommendations of the Standing Committee on Travel Medicine (StAR) of the DTG. (PDF) German Society for Tropical Medicine and International Health e. V., June 2019.
- ↑ Mark Denbeaux, Sean Camoni, Brian Beroth, Meghan Chrisner, Chrystal Loyer, Kelli Stout, Paul Taylor: Drug abuse. An exploration of the government's use of mefloquine at Guantanamo. ( Memento of February 15, 2012 in the Internet Archive ) (PDF; 1.2 MB). Published on the Seton University School of Law website. December 2010.
- ↑ Elke Brüser: Torture in Guantanamo. "Pharmacological Waterboarding" . In: Süddeutsche Zeitung. December 15, 2010.
- ↑ Anna Gabriel: Torture in Guantánamo with drugs? In: The press. December 18, 2010.
- ^ Andreas Förster: Guantanamo prisoners as guinea pigs . In: Berliner Zeitung , March 3, 2011.
- ^ RL Nevin: Mass administration of the antimalarial drug mefloquine to Guantánamo detainees: a critical analysis. In: Trop Med Int Health. August 12, 2012 doi: 10.1111 / j.1365-3156.2012.03063.x . PMID 22882560 .
- ^ Inge Hoeger (Die Linke): Written question: Compensation for Bundeswehr soldiers because of psychological side effects of the malaria drug Lariam ®. (PDF) German Bundestag. 18th legislative term. Printed matter 18/115. November 29, 2013, pp. 43-44.
- ^ Penny Wright: Mefloquine inquiry in the Australian Senate. September 18, 2012.
- ↑ Rolf Käppeli: Kamboriam. A report. In the malaria jungle of Cambodia and Switzerland. With an afterword by Adolf Muschg. Zug: Balmer Verlag 1995. ISBN 978-3-85548-037-1
- ^ Law & Order: New York. Episode Guide: Follow Side Effects. Universal TV 2005.
- ^ Christian Kracht, David Woodard: Five Years. Correspondence 2004-2009. Hanover: Wehrhahn Verlag 2001. pp. 18-19. ISBN 978-3-86525-235-7 . Quote: "[O] ne needs to take extraordinary little white pills called 'Lariam', which, once ingested, tend to creat havoc with one's emotions. Upon visiting Somalia in the early nineties, I found the little buggers down daily and could not stop crying and falling into thoughts of deepest chemical depression. I would rather chance malaria than have to take those pills again. "
- ↑ Ann Patchett: River of Miracles. Translated by Werner Loch-Lawrence. Berlin: Bloomsbury 2012. ISBN 978-3-8270-1056-8 .
- ^ David Stuart MacLean: The Answer to the Riddle is Me. A memoir to Amnesia. Boston: Houghton Mifflin Harcourt 2014. ISBN 978-0-547-51927-2
- ^ Robert Löhr (screenplay), Markus Sehr (director): The Institute - Oasis of Failure. Episode "The Exorcism of Anneliese E." Novafilm 2019.
- ↑ Smo: Personal details: shy colleagues . In: Spiegel Online . tape 42 , October 14, 2017 ( spiegel.de [accessed November 12, 2019]).