Plasmodium falciparum
| Plasmodium falciparum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

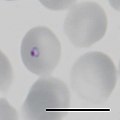
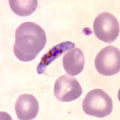
Different forms of Plasmodium falciparum on stained blood smear |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Plasmodium falciparum | ||||||||||||
| ( Welch ), 1897 |
Plasmodium falciparum is a type of unicellular parasite from the genus Plasmodium , which is ofparamount importanceas a pathogen causing the life-threatening malaria tropica in humans. The World Health Organization estimates that around 247 million malaria cases resulted in nearly 881,000 deaths in 2006, the vast majority of them from Plasmodium falciparum . Other authors estimate that in 2002 there were 515 million cases of malaria per year due to Plasmodium falciparum .
Plasmodium falciparum differs in many features from other malaria pathogens and is therefore classified together with a chimpanzee-infecting parasite in its own subgenus Laverania . Man is the only natural host. Plasmodium falciparum is transmitted by female mosquitoes of the genus Anopheles , in which sexual reproduction also takes place. Today, the parasite is mainly found in tropical countries, while the vast majority of cases of tropical malaria are in sub-Saharan Africa.
Plasmodium falciparum has a complex life cycle, in which, after infection, the first proliferation in the liver is followed by strong proliferation in the blood. The pathogen is particularly dangerous due to certain peculiarities of the reproduction in the blood, such as the often high number of parasites, the adherence of infected blood cells to the walls of blood vessels and the formation of rosettes between infected cells and other blood cells. Plasmodium falciparum has developed special molecular mechanisms for these properties, which also serve to evade the effects of the human immune system .
Discovery and Description
history
Plasmodium falciparum was first identified in 1880 by Alphonse Laveran in a blood smear from a soldier who had malaria in Algeria. The exflagellation process observed by Laveran in the unfixed microscopic specimen, in which a microgametocyte, a sexual form of the parasite, quickly turns into several mobile male gametes , was evidence that the particles observed in the blood were not artifacts, but life forms of one unicellular blood parasites.
Laveran assumed only one type of malaria pathogen in humans, which he called Oscillaria malariae . Other authors suspected different pathogens for the different forms of malaria. Ettore Marchiafava and Angelo Celli introduced the generic name Plasmodium for the malaria pathogens in 1885 . The pathogen causing malaria tropica was named Haematozoon falciparum by William Henry Welch in 1897 after reviewing the literature . The epithet falciparum is derived from the Latin word falx for sickle and refers to the characteristic sickle-shaped shape of the gametocytes. Between 1885 and 1897 a number of other names were suggested by different authors; contrary to the priority rule , the name Plasmodium falciparum prevailed in the literature . In 1954 the epithet falciparum was declared valid by the ICZN , both in the usual combination Plasmodium falciparum and in the uncommon name Laverania falciparum .
morphology
Like all plasmodia, P. falciparum occurs in different stages of development, of which the blood types are the best known and most important for diagnosis. The mosquito-derived sporozoites are sickle-shaped and usually 10.5 to 12 micrometers long. Liver schizonts, the first form of development in humans, are initially round to oval, in later stages they often have an irregular, lobed shape and reach a size of up to 60 micrometers. They contain tens of thousands of merozoites , each about 0.7 micrometers in diameter.
When the blood replicates in erythrocytes , a high level of parasitemia is just as typical for this pathogen as the frequent absence of later forms of development in the peripheral blood; in the blood smear usually only ring shapes and, after a long illness, mature gametocytes are observed. In the course of development in the erythrocytes, ring-shaped trophozoites are first formed, which are smaller than other human malaria pathogens. Infection of a blood cell by multiple merozoites is common. Erythrocytes with older, larger ring shapes often show a typical Maurerian stain after staining . In the further course of development, the parasites become larger and more compact without forming amoeboid forms, as is the case with some other malaria pathogens. In the stained blood smear, the cytoplasm is stained deep blue at this stage, and malaria pigment can be seen for the first time at this stage.
Later forms of development are usually only observed in small numbers in the blood smear. Multiple division turns the trophozoite into a schizont that enlarges and fills most of the erythrocytes. Mature blood schizonts contain 8 to 20, typically 16, merozoites. The sometimes mentioned number of up to 32 merozoites is probably due to a multiple infection with two schizonts in one erythrocyte. In P. reichowi , which is otherwise morphologically hardly distinguishable from P. falciparum , the number of merozoites is limited to 10 to 12.
Immature gametocytes are also relatively rarely observed in the peripheral blood. Characteristic of the species is the sickle-shaped shape of the mature gametocytes, a property that was only observed in the subgenus Laverania in Plasmodia that infect mammals . The macrogametocytes are relatively slim, the cytoplasm is clearly blue after staining, and the nucleus is compact. The microgametocytes, on the other hand, are more plump in shape, the cytoplasm is light blue after coloring, the cell nucleus is larger and less compact.
As apicomplexa , plasmodia have a special organelle , the apicoplast . This plastid , which is believed to have arisen from the endosymbiosis of red algae , has lost its photosynthesis ability , but is vital for plasmodia in the gametocytogenesis stage (the asexual developmental stages in the erythrocytes and in the liver). Therefore, the apicoplast is an attractive target for anti-malarial drugs.
- Forms of development in the stained blood smear
Systematics
The original host of P. falciparum is the western gorilla ( Gorilla gorilla ). According to a study published in 2010, the pathogen was transmitted once from a gorilla to a human. In 2019, the transition to humans as a result of a gene transfer from Plasmodium adleri to Plasmodium falciparum around 50,000 years ago was described.
In contrast to the other malaria pathogens in humans, P. falciparum is not classified in the subgenus Plasmodium , but in its own subgenus Laverania . The Laverania include a total of six different species of Plasmodium , which infect the common chimpanzee ( Pan troglodytes ) and the western gorilla, but not the eastern gorilla ( Gorilla beringei ) and the bonobo ( Pan paniscus ). The morphologically almost identical P. reichowi , a chimpanzee parasite, has long been suspected of being the origin of P. falciparum .
Although morphological, immunological and genetic differences can often be determined between different isolates of P. falciparum , no taxonomic differentiation of the species, for example into subspecies, has been able to establish itself, since the different characteristics are not constant and different enough to justify a corresponding delimitation. In population genetic studies, surprisingly few polymorphisms were found in parts of the genome and in the mitochondrial genome of P. falciparum . The currently most plausible explanation for this is a possible rapid expansion of a small parasite population in Africa around 10,000 years ago. This expansion into a genetic bottleneck could have coincided with changes in the ecology of humans, the only mammalian host of the parasite, in the Neolithic revolution .
Spread and hosts
distribution
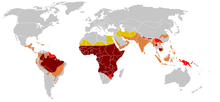
Plasmodium falciparum can occur in tropical and subtropical areas. Today P. falciparum is mainly found in the tropics, especially in Africa south of the Sahara. Other focuses are in parts of South and Southeast Asia and Papua New Guinea as well as in the Amazon basin. Overall, more than 40% of the world population lives in regions at risk of infection by P. falciparum . As a general rule, it is often stated that the parasites are transmitted in the tropics up to an altitude of 1,500 meters, but there are also proven cases of transmissions from 2,600 to 2,800 meters.
Until the middle of the 20th century, the parasite was also found in the Mediterranean region , in Europe, for example, in Spain, Italy and the Balkans, as well as in the southeastern United States. In contrast to other malaria pathogens , P. falciparum was never native to Central Europe . A natural cycle of spreading from person to person by mosquitoes is limited here to extremely rare individual cases, for which an undetected parasite carrier, a suitable Anopheles population and a prolonged heat period favoring the multiplication of the parasite in the mosquito must come together.
Through genetic analysis it was found that the pathogens in South America originate from Africa south of the Sahara, i.e. were introduced with the Atlantic slave trade . The authors suspect that the two main genetic strands in South America (a northern and a southern) were introduced independently of one another.
Mammalian hosts
Under natural conditions, only humans are infected with P. falciparum ; other relevant reservoir hosts are not known. Experimentally, chimpanzees can be infected with P. falciparum by mosquitoes ; however, development is limited to the reproduction phase in the liver. If the spleen is removed from a chimpanzee, the parasites can also multiply in the blood, but no mature gametocytes are observed. Also Gibbons can be infected, but also produce no mature gametocytes.
Overnight monkeys from South America, which can be experimentally infected with P. falciparum , are of great importance for malaria research . In the gray-handed night monkey , a complete development cycle even succeeds in a reproducible manner with the transmission of the parasite from monkey to monkey by mosquitoes. Since 1976, this animal model has been supplemented by the important possibility of studying P. falciparum in continuous cell culture in human erythrocytes.
Insect hosts
A large number of mosquito species are able to transmit P. falciparum . Garnham (1966) names 66 species from the genus Anopheles as suitable vectors . Anopheles gambiae sensu strictu is of great epidemiological importance in tropical Africa, as this mosquito prefers to bite people and thus promotes the spread of the parasite. Suitable vectors include Anopheles atroparvus , Anopheles messeae and possibly Anopheles plumbeus also occurring in Europe. Not all mosquito species should be suitable for all P. falciparum parasites; African parasites should not be found in European mosquitoes of the species Ano. atroparvus multiply.
Life cycle
The life cycle of P. falciparum, with its obligatory host change between Anopheles mosquitoes and humans, is similar to the cycle of other plasmodia, but has a number of peculiarities compared to the other malaria pathogens in humans, some of which have important consequences for the course of the disease.
Infection and multiplication in the liver
The sporozoites enter the human bloodstream through infected mosquitoes, migrate from there to the liver and penetrate hepatocytes , in which they reproduce asexually through schizogony . The incubation period of this liver phase is at least five and a half days. The liver schizonts each produce up to 40,000 merozoites, which are released into the bloodstream and attack erythrocytes of all stages of maturity. In contrast to some other malaria pathogens, P. falciparum only has a single cycle of reproduction in the tissue; permanent forms of the parasite in the liver were not observed.
Increase in blood
Parasites are microscopically detectable in the blood seven days after infection at the earliest, typically the prepatency is around eleven days. Another asexual reproduction takes place in the erythrocytes (here the transport protein anion exchanger 1 plays the role of the entry point). The generation time for the replication in the erythrocytes is on average 48 hours, but a synchronization of the replication with a pronounced fever cycle is rare, in contrast to the other malaria pathogens in humans. In the erythrocyte cycle of P. falciparum in the peripheral blood, infected cells are only detected in large numbers in the first 24 hours; schizonts are only observed there relatively rarely. This is because the infected erythrocytes with maturing schizonts remain in postcapillary venules in various organs, where they attach to the endothelium of the blood vessels in order to avoid elimination in the spleen . A similar behavior is shown by many animals infecting Plasmodium species, particularly marked, it is in some pathogens of monkey malaria like P. coatneyi and the closely related P. falciparum related P. reichenowi . Another characteristic of P. falciparum is the formation of rosettes between infected erythrocytes and uninfected erythrocytes. The combination of infected erythrocytes adhering to the capillary walls and the formation of rosettes can block fine capillaries in vital organs such as the brain and thus impair the oxygen supply. This can contribute to the often fatal course of the severe malaria tropica.
Sexual reproduction
A few plasmodia develop in the erythrocytes into sex forms, the gametocytes. These are typically observed eight to eleven days after the first appearance of the asexual forms in the blood; the development of gametocytes is very slow compared to other types of plasmodia. It is also unusual that only mature gametocytes are observed in the peripheral blood. Gametocytes mature in the bone marrow , where erythrocytes infected with developing gametocytes are bound by cell adhesion molecules . The mature microgametocytes and macrogametocytes can be ingested by mosquitoes with a blood meal and start a new development cycle in the insect's intestine. After the gametes fuse, new sporozoites are formed in the intestine, which migrate to the mosquito's salivary gland, from where they can be transferred to a new host. The development time of P. falciparum in mosquitoes is around 23 days at 20 ° C, around ten days at 25 ° C and around nine days at 30 ° C. In the mosquito's salivary gland, the sporozoites retain their infectivity for 40 to 55 days.
Molecular Properties
Due to its outstanding medical importance as a pathogen, P. falciparum has been extensively investigated in order to identify new approaches for prophylaxis and therapy. The focus was on the mechanisms of the particular pathophysiology of tropical malaria, but also on the mechanisms with which P. falciparum undermines the activity of the patient's immune system.
Parasite-Host Interactions
Parasites living inside host cells, such as plasmodia, generally need host factors in order to recognize suitable cells and to penetrate them. P. falciparum uses a variety of proteins at various stages to interact with human cells.
In order to penetrate erythrocytes, the merozoites of the plasmodia need certain receptors on the cell surface of the host cells. P. falciparum can use several routes for this and switch between them if necessary. Important receptors here are the glycophorins on the erythrocytes and the anion exchanger 1 . These glycoproteins must have a specific glycosylation pattern in order to enable binding of the parasite protein EBA175 and successful infection. The dependence on this glycosylation pattern is also one of the reasons for the parasite's high host specificity.
The so-called PfEMP1 ( P. falciparum erythrocyte membrane protein 1) plays a central role in the sequestration of infected erythrocytes in the blood vessels of the organs, which is typical for P. falciparum . The protein is produced by the parasite and presented on the surface of the infected red blood cell. There, PfEMP1 can bind to various receptors such as CD36 on endothelial cells in the blood capillary and, through this binding, attach the infected erythrocytes to the blood vessel wall. A certain form of PfEMP1 can bind to chondroitin sulfate in the placenta and thus contribute to a problematic course of the disease during pregnancy. Finally, PfEMP1 can bind to the complement receptor 1 on erythrocytes and initiate rosette formation of infected and non-infected erythrocytes via this. All of these interactions with host receptors emanating from PfEMP1 presumably play a role in the course of the disease.
Antigen variability
Like all plasmodia, P. falciparum uses a number of mechanisms to evade the host's immune defense. Here too the PfEMP1 plays a central role. Since PfEMP1 is present on the cell surface of the erythrocytes, antibodies against the parasite protein are formed by the patient . In order to avoid this immune reaction, P. falciparum can exchange the PfEMP1. PfEMP1 is encoded by a multigene family with around 60 var genes, of which only one is active at any one time. If the active var gene is changed and another PfEMP1 is produced, the patient's acquired immunity often comes to nothing. In malaria endemic areas, it takes many years for people to acquire at least partial immunity to the various parasite antigens. Different PfEMP1 proteins from different var genes can bind a different spectrum of endothelial receptors. It is not certain whether certain PfEMP1 proteins and their binding to organ-specific receptors are responsible for particularly severe disease courses such as cerebral malaria. Currently, the role of the placenta-binding form of PfEMP1 in the course of malaria during pregnancy is best understood. With the exception of P. falciparum , the var gene family was only found in the closely related P.richowi .
Genome
To better understand the biology of the parasite, the genome was completely sequenced in 2002. It comprises around 23.3 mega base pairs on 14 chromosomes , which contain around 5,400 genes, the function of which, however, is often unknown. What is unusual compared to other plasmodia is a very low GC content of less than 20%, an extreme value within eukaryotes. As with all plasmodia, the genome is rich in repetitive sequences . Many of the gene families responsible for the antigen variability in P. falciparum are located on the telomeres of the chromosomes and are specific to the Laverania subgenus .
Individual evidence
- ↑ WHO: World Malaria Report 2008 (PDF)
- ↑ RW Snow, CA Guerra, AM Noor, HY Myint, SI Hay: The global distribution of clinical episodes of Plasmodium falciparum malaria. In: Nature . 434 (7030), Mar 10, 2005, pp. 214-217. PMID 15759000
- ↑ B. Striepen: The apicoplast: a red alga in human parasites. In: Essays Biochem. 51, 2011, pp. 111-125. PMID 22023445
- ↑ M. Kalanon, GI McFadden: Malaria, Plasmodium falciparum and its apicoplast. In: Biochem Soc Trans. Volume 38, No. 3, June 2010, pp. 775-782. PMID 20491664
- ↑ JD Wiley et al: Isoprenoid precursor biosynthesis is the essential metabolic role of the apicoplast during gametocytogenesis in Plasmodium falciparum. In: Eukaryote. Cell. Volume 14, No. 2, February 2015, pp. 128-139. PMID 25446055
- ↑ J. Wiesner, H. Jomaa: Isoprenoid biosynthesis of the apicoplast as drug target. In: Curr Drug Targets. Volume 8, No. 1, January 2007, pp. 3-13. PMID 17266527
- ↑ A. Mukherjee, GC Sadhukhan: Anti-malarial Drug Design by targeting Apicoplasts: New Perspectives. In: J Pharmacopuncture. Volume 19, No. 1, March 2016, pp. 7-15. PMID 27280044
- ↑ a b Liu Weimin et al: Origin of the human malaria parasite Plasmodium falciparum in gorillas. In: Nature. Volume 467, No. 7314, 2010, pp. 420-425.
-
↑ Francis Galaway et al .: Resurrection of the ancestral RH5 invasion ligand Provides a molecular explanation for the origin of P. falciparum malaria in humans. In: PLoS Biology. Volume 17, No. 10, e3000490, doi: 10.1371 / journal.pbio.3000490 .
Resurrection of 50,000-year-old gene reveals how malaria jumped from gorillas to humans. On: eurekalert.org from October 15, 2019. - ↑ DL Hartl: The origin of malaria: mixed messages from genetic diversity. In: Nat Rev Microbiol . Volume 2, No. 1, 2004, pp. 15-22. PMID 15035005
- ↑ SI Hay, CA Guerra, PW Gething, AP Patil, AJ Tatem, AM Noor, CW Kabaria, BH Manh, IR Elyazar, S. Brooker, DL Smith, RA Moyeed, RW Snow: A World Malaria Map: Plasmodium falciparum Endemicity in 2007. In: PLoS Med . Volume 6, No. 3, 24 Mar 2009, p. E48. PMID 19323591
- ^ A. Krüger, A. Rech, XZ Su, E. Tannich: Two cases of autochthonous Plasmodium falciparum malaria in Germany with evidence for local transmission by indigenous Anopheles plumbeus. In: Trop Med Int Health. Volume 6, No. 12, 2001, pp. 983-985. PMID 11737834
- ↑ Malaria: Spread through slave trade. science.orf.at, December 27, 2011.
- ^ S. Herrera, BL Perlaza, A. Bonelo, M. Arévalo-Herrera: Aotus monkeys: their great value for anti-malaria vaccines and drug testing. In: Int J Parasitol. Volume 32, No. 13, Dec 4, 2002, pp. 1625-1635. PMID 12435447
- ↑ W. Trager, JB Jensen: Human malaria parasites in continuous culture. In: Science . Volume 193, 1976, pp. 673-675. PMID 781840 .
- ↑ P. Alano: Plasmodium falciparum gametocytes: still many secrets of a hidden life. In: Mol Microbiol. Volume 66, No. 2, 2007, pp. 291-302. PMID 17784927
- ↑ D. Chattopadhyay, J. Rayner, AM McHenry, J. H Adams: The structure of the Plasmodium falciparum EBA175 ligand domain and the molecular basis of host specificity. In: Trends Parasitol. Volume 22, No. 4, 2006, pp. 143-145. PMID 16497558
- ↑ a b S. A. Kyes, SM Kraemer, JD Smith: Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. In: Eukaryot Cell. Volume 6, No. 9, Sep 2007, pp. 1511-1520. PMID 17644655
- ↑ MJ Gardner et al: Genome sequence of the human malaria parasite Plasmodium falciparum. In: Nature. Volume 419, October 3, 2002, pp. 498-511.
- ↑ TW Kooij, CJ Janse, AP Waters: Plasmodium post-genomics: better the bug you know? In: Nat Rev Microbiol. Volume 4, No. 5, 2006, pp. 344-357. PMID 16582929
literature
- G. Robert Coatney, William E. Collins, McWilson Warren, Peter G. Contacos: The primate malarias. US National Institute of Allergy and Infectious Diseases, Bethesda 1971, Chapter 22, p. 263 ff .: Plasmodium falciparum (PDF)
- Percy Cyril Claude Garnham: Malaria Parasites and other Haemosporidia. Blackwell Scientific Publications, Oxford 1966, ISBN 0-632-01770-8 , Chapter XIV, pp. 357 ff .: Plasmodium falciparum and Plasmodiumreichenowi.