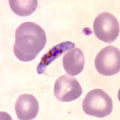
Plasmodium
| Plasmodium | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

Schizont of Plasmodium malariae , the type species of the genus, in stained blood smear |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Plasmodia | ||||||||||||
| Marchiafava & Celli , 1885 |
The genus Plasmodium belongs to the Haemospororida within the group of Apicomplexa . Plasmodium are unicellular parasites that have great medical importance because the pathogens of malaria belong to this genus. The approximately 200 species of the genus parasitize on a large number of terrestrial mammals , reptiles and birds . All species change host ; they are usually transmitted to vertebrates by blood-sucking mosquitoes . Plasmodia occur practically worldwide, but the malaria pathogens that are dangerous for humans are now limited to tropical and subtropical countries.
History and description
Plasmodia as the causative agent of malaria was first described in Algeria by Alphonse Laveran in 1880 under the name Oscillaria malariae . Shortly afterwards, Danilewsky discovered various blood parasites, including plasmodia, in birds in the Ukraine. The now recognized generic name Plasmodium was proposed in 1885 by the Italians Marchiafava and Celli.
Like all Haemospororida, the plasmodia parasitize first in the tissue, then in the blood cells of vertebrates. There they also develop gender forms, so-called gametocytes, a distinction being made between microgametocytes and macrogametocytes. Only microgametes carry a single scourge during their short existence . Like most apicomplexa, plasmodia have an apicoplast, a plastid without chlorophyll that can only be seen under an electron microscope , which is not capable of photosynthesis, but in which other essential metabolic processes take place.
The genus Plasmodium differs from other genera of the Haemospororida in that the parasites multiply asexually in erythrocytes in the blood of the vertebrates through a multiple division, the so-called schizogony , while other parasites only develop gametocytes there. In schizogony, the erythrocytes develop from an initially ring-shaped, later amoeboid trophozoite into a schizont with a number of merozoites typical for the respective species , which are ultimately released into the blood and infect further erythrocytes there. Another feature that distinguishes Plasmodia from many other haemospororidae is the formation of a pigment in the erythrocytes called haemozoin . This pigment is a breakdown product of hemoglobin , which is caused by incomplete digestion by the parasites. The plasmodia in the erythrocytes essentially feed on hemoglobin and glucose.
- Forms of development of different types of plasmodia
Spindle-shaped Plasmodium sporozoite in intestinal epithelial cells from Anopheles stephensi . False color EM recording
Spread and hosts
Plasmodia occur on all continents with the exception of the polar regions. Various mammals , birds and reptiles are infected . The vectors are almost exclusively female mosquitoes , other vectors were only found in reptile-infecting species.
Plasmodia infecting mammals
Plasmodia infect a wide variety of mammals, particularly primates and rodents . Plasmodia are very rare in other mammals. Most parasites show a high host specificity. Their range today are the tropics and subtropics; however, human malaria was also widespread in Europe and North America until the 20th century.
A monograph from 1971 names 24 described species that infect primates (excluding lemurs). The classic malaria pathogens in humans are Plasmodium falciparum , Plasmodium malariae , Plasmodium ovale and Plasmodium vivax . For these species, humans are seen as the only epidemiologically relevant reservoir. But also Plasmodium knowlesi , a malaria pathogen in macaques in Southeast Asia, is important as a pathogen in humans. Other monkey malaria pathogens that can infect humans without having any medical significance are P. brasilianum , P. cynomolgi and P. inui . To the malaria pathogens in humans there are partly closely related, partly practically identical pathogens in monkeys, for example P. falciparum and the chimpanzee infecting P. reichowi , P. malariae and the Brazilian New World monkey infecting P. brasilianum as well as P. vivax and the also Brazilian New World monkey infecting P. simium .
In addition to these primate plasmodia, which have been extensively described because of their medical importance, there are a number of other, in part little studied species from the subgenus Vinckeia with 21 species described in 1978. These infect a number of rodents, including mice , flying squirrels and porcupines , as well as bats and lemurs . A few ruminants are also attacked, such as the water buffalo , the duiker , the stag pig and the white-tailed deer . Plasmodia, which infect various African thicket rats and acacia mice , have been important model organisms for malaria research since the 1950s , since house mice , and sometimes laboratory rats , can also be infected with P. berghei , P. chabaudi and P. yoelii in the laboratory . None of the plasmodia infecting mammals play a role as pathogens in domestic animals and farm animals.
The plasmodia that infect mammals are transmitted exclusively by female mosquitoes of the genus Anopheles .
Plasmodia infecting birds
Plasmodia cause avian malaria in a large number of bird species worldwide. The host specificity of many species is often significantly lower than that of the plasmodia that infect mammals; some parasites can infect dozens of different bird species. Avian plasmodia are common on all continents. Most infections in wild birds are mild and asymptomatic. However, infections caused by the widespread Plasmodium relictum , for example, can lead to numerous deaths. A 2005 monograph lists 38 described species that infect birds.
In poultry farming in tropical countries, poultry malaria , caused by P. gallinaceum , P. juxtanucleare and P. durae , can lead to high losses in chickens or turkeys. Plasmodia that infect birds have long been important models for malaria research. In 1898, Ronald Ross identified the life cycle of plasmodia in mosquitoes using the example of a bird parasite. Until the 1940s, plasmodia infecting birds were important model organisms for research into new drugs for the treatment of malaria in humans.
Plasmodia that infect birds are mainly transmitted by mosquitoes of the genera Culex , Culiseta and Aedes , and partly also by Anopheles , Psorophora and Mansonia .
Plasmodia infecting reptiles
Many different types of plasmodia infect reptiles. The vast majority of the hosts of these parasites are lizards , only three species have been found in snakes . The host specificity is high. A literature review from 1988 names 69 species described. Most of the species were found in Latin America and Africa, with a few also found in the United States, Southeast Asia, Australia, and New Zealand.
For many of these parasites the vectors are not known. In addition to mosquitoes such as Culex , which transmit P. floridense , for example , other two-winged birds were also investigated as vectors. It could be shown that P. mexicanum can be transmitted by sandflies of the genus Lutzomyia .
Life cycle
All plasmodia have a complex life cycle with an obligatory host switch between insects, in which sexual reproduction occurs, and vertebrates, in which asexual reproduction takes place. In the vertebrates, a first, mostly symptom-free phase of exoerythrocytic proliferation in the tissue must be distinguished from a second proliferation phase in the erythrocytes in the blood, accompanied by symptoms of the disease.
Reproduction in vertebrates: exoerythrocytic phase
The life cycle begins with sporozoites that get into the body of a vertebrate via infected mosquitoes with the saliva. The spindle-shaped sporozoites penetrate host cells and first multiply in the tissue in an exoerythrocytic phase through schizogony . In mammals, the mosquitoes inject the parasites into the dermis , from where they actively migrate to blood vessels and are transported through these to the liver within minutes. There they probably migrate via Kupffer cells into hepatocytes , in which, depending on the species, they multiply massively in two to 16 days. In primates, tissue schizonts can grow to over 80 micrometers in size and contain tens of thousands of merozoites. Some of the Plasmodium species can develop dormant forms in the liver, so-called hypnozoites , which can relapse after the disease has healed. This is known, for example, for P. cynomolgi , P. fieldi , P. ovale , P. simiovale and P. vivax . Avian and reptilian parasites are found in a number of tissues at this stage. The bird parasite P. relictum, for example, reproduces exoerythrocytically in the skin, liver, spleen and bone marrow. Other species, including reptile-infecting species such as P. mexicanum , have been found in endothelial cells and in hematopoietic cells. In the case of many bird and reptile parasites, however, the location of exoerythrocytic reproduction is not known. In mammals, the exoerythrocytic phase is usually asymptomatic; in bird parasites, serious illnesses can occur even at this stage. Massive infection of the bone marrow with P. elongatum can lead to deaths, as can exoerythrocytic schizogonia of P. gallinaceum in the capillary endothelium of various organs, especially the brain.
Multiplication in the vertebrate: erythrocytic phase
The tissue schizonts each produce large numbers of small merozoites that are released and, in a second phase of infection, attack erythrocytes in the blood, in which further asexual reproduction takes place through schizogony. This increase in the blood, which takes one to three days depending on the species, is the cause of the symptoms of malaria. In primates, reproduction often takes place synchronized in this phase, so that many mature blood schizonts simultaneously release merozoites, which is accompanied by the release of toxic substances. This leads to the well-known periodicity of the fever attacks in malaria disease. In other mammals and birds, the synchronicity of development is less pronounced; in reptiles it is not observed at all.
A few plasmodia develop in the erythrocytes into sex forms, the gametocytes. These are essential to complete the life cycle and to enable further spread by mosquitoes.
Multiplication in insects
The gametocytes can be ingested by mosquitoes with a blood meal and start a new development cycle in the insect's intestine. In a process called exflagellation, the microgametocytes in the mosquito's intestine divide into microgametons within a few minutes, which carry a single flagella and are accordingly mobile. By fusing a microgamete with a macrogamete, a zygote is formed, in which a meiotic division then takes place. The zygote develops into a mobile ookinete in the intestine, which transforms into an oocyst in the intestinal wall. Depending on the type and temperature, many new sporozoites are formed within about one to four weeks through multiple cell division. These eventually migrate into the mosquito's salivary gland, where they mature into highly infectious forms. From there they can be transferred to a new vertebrate host.
Molecular Properties
Due to their great medical importance, especially plasmodia that infect mammals have been intensively studied using molecular biology methods in order to obtain new approaches for the prophylaxis and therapy of malaria.
Plasmodia have developed complex mechanisms to circumvent an acquired host immunity. These include highly repetitive protein sequences and mechanisms of antigen variability with which the parasites regularly exchange antigens on the cell surface. Not only does this prevent full immunity from disease, it also makes vaccine development difficult .
The metabolism of the plasmodia has a number of peculiarities that are used as targets for medicinal substances . Chloroquine and related substances inhibit the formation of hemozoin, atovaquone and related substances inhibit mitochondrial respiration, folate antagonists such as proguanil inhibit folate biosynthesis in the cytoplasm, and various antibiotics such as tetracyclines inhibit metabolic processes in the apicoplast. Substances derived from artemisinin , all of which are characterized by an endo peroxide group , are currently of great therapeutic importance . These substances could act both by generating free radicals and by inhibiting a Ca 2+ - ATPase in the parasites.
Complete genome sequences have been determined from a growing number of Plasmodia , such as P. falciparum , P. vivax , P. knowlesi and various mouse malaria pathogens. All genomes examined so far consist of 14 chromosomes with 23–27 million base pairs and approximately 5,500 genes . However, the function of almost half of the genes is unknown.
Systematics
External system
In the systematics of eukaryotes according to Adl et al. the genus Plasmodium is directly subordinate to the Haemospororida . The only other genus mentioned in this classification besides Plasmodium , Mesnilium parasitizes in fish. The Haemospororida in turn belong to the Aconoidasida with the Piroplasmorida as a sister group . The subdivision of the Aconoidasida as in Adl et al. is supported by various molecular analyzes including the fully sequenced genomes . The systematics according to Adl et al., 2005 at a glance:
- Apicomplexa Levine, 1980, emend. Adl et al., 2005
- Aconoidasida Mehlhorn, Peters & Haberkorn, 1980
- Haemospororida Danilewsky, 1885
- Plasmodium Marchiafava & Celli, 1885
- Mesnilium Misra, Haldar & Chakravarty, 1972
- Piroplasmorida Wenyon, 1926
- Haemospororida Danilewsky, 1885
- Aconoidasida Mehlhorn, Peters & Haberkorn, 1980
However, the system of Adl et al. incomplete, since many genera that have long been undoubtedly assigned to the Haemospororida are not mentioned. In older systematics the Haemospororida was subordinated to the family Plasmodiidae with ten genera, which in addition to Plasmodium also include Haemoproteus , Hepatocystis and Leucocytozoon . These four genera make up 95% of all species within the Haemospororida. The close relationship of these genera is well established by molecular studies; no DNA sequences are yet available from other genera.
Internal system
Traditionally, the taxonomy of plasmodia is based on morphology, life cycle and host specificity, a new system based on phylogenetic criteria is pending. The genus currently comprises around 200 species, which are divided into up to 15 subgenera, of which three infect mammals, five birds and seven reptiles. This systematics, along with species of medical, veterinary and research significance, has the following structure:
- Genus Plasmodium Marchiafava & Celli, 1885
- Subgenus Asiamoeba Telford, 1988 (lizards)
- Subgenus Bennettinia Valkiūnas, 1997 (birds)
- Plasmodium juxtanucleare Versiani & Gomes, 1941; Chicken malaria pathogen in Asia, Africa and South America
- Subgenus Carinamoeba Garnham, 1966 (lizards)
- Subgenus Garnia Lainson, Landau & Shaw, 1971 (lizards)
- Subgenus Giovannolaia Corradetti, Garnham & Laird, 1963 (birds)
- Plasmodium durae Herman, 1941; Malaria pathogen in turkeys in Africa
- Subgenus Haemamoeba Grassi and Feletti, 1890 (birds)
- Plasmodium gallinaceum Brumpt, 1935; Chicken malaria pathogen in Asia and Africa
- Subgenus Huffia Corradetti, Garnham & Laird, 1963 (birds)
- Subgenus Lacertaemoba Telford, 1988 (lizards)
- Subgenus Laverania Bray, 1963 (humans and chimpanzees)
- Plasmodium falciparum (Welch, 1897); Malaria tropica pathogenin humans
- Subgenus Novyella Corradetti, Garnham & Laird, 1963 (birds)
- Subgenus Ophidiella Garnham, 1966 (snakes)
- Subgenus Plasmodium Bray, emend 1963. Garnham, 1964 (primates)
- Plasmodium knowlesi Sinton & Mulligan, 1932; Malaria pathogen in macaques and humans in Southeast Asia
- Plasmodium malariae (Grassi & Feletti, 1890); Quartana malaria pathogenin humans
- Plasmodium ovale Stephens, 1922; Tertiana malaria pathogenin humans
- Plasmodium vivax (Grassi & Feletti, 1890); Tertiana malaria pathogen in humans
- Subgenus Paraplasmodium Telford, 1988 (lizards)
- Subgenus Sauramoeba Garnham, 1966 (lizards)
- Subgenus Vinckeia Garnham, 1964 (mammals)
- Plasmodium berghei Vincke & Lips, 1948
- Plasmodium chabaudi Landau, 1965
- Plasmodium yoelii Landau, Michel & Adam, 1968
The phylogenesis of plasmodia and other apicomplexes has been investigated for years using methods of DNA sequence analysis without a conclusive picture so far. According to the current status, the genus Plasmodium is probably paraphyletic to the little-studied genus Hepatocystis , although Hepatocystis has a significantly different life cycle and is also transmitted by other insects than Plasmodium .
Within the genus, the parasites infecting mammals form a clade together with representatives of the Hepatocystis genus . It is considered certain that the transition to mammals occurred only once in evolution. The thesis, which has been discussed since the 1990s, that the subgenus Laverania with the important malaria pathogen P. falciparum emerged from a bird malaria parasite in more recent times, is no longer supported. The parasites infecting reptiles and birds together form another clade, and there is evidence of multiple changes of parasites between reptiles and birds as hosts. The birds infecting subgenera are Haemamoeba , Huffia and Bennettinia probably monophyletic , Giovannolaia and Novyella is not. Overall, too little molecular data is currently available to revise the taxonomy of Plasmodia or Haemospororida at all on this basis.
Reporting requirement
In Germany, the direct or indirect detection of Plasmodium sp. Not notifiable by name according to § 7 paragraph 3 of the Infection Protection Act (IfSG). The laboratories etc. are obliged to report with regard to the detection of the pathogen ( § 8 IfSG).
In Switzerland, the positive laboratory results for Plasmodium spp. Notifiable for laboratories according to the Epidemics Act (EpG) in conjunction with the Epidemics Ordinance and Appendix 3 of the EDI Ordinance on the reporting of observations of communicable diseases in humans .
Individual evidence
- ↑ G. Robert Coatney, William E. Collins, McWilson Warren and Peter G. Contacos: The primate malarias. Bethesda: US National Institute of Allergy and Infectious Diseases, 1971. Online .
- ↑ R. Killick-Kendrick, W. Peters (Ed.): Rodent Malaria. London: Academic Press, 1978. ISBN 0-12-407150-3 .
- ^ Garnham PC, Kuttler KL. A malaria parasite of the white-tailed deer (Odocoileus virginianus) and its relation with known species of Plasmodium in other ungulates. In: Proc R Soc Lond B Biol Sci. 1980 Jan 17; 206 (1165): 395-402. PMID 6102388 .
- ↑ Gediminas Valkiūnas: Avian Malaria Parasites and Other Haemosporidia. Boca Raton: CRC Press, 2005. ISBN 0-415-30097-5 .
- ^ The Merck Veterinary Manual: Plasmodium Infection .
- ↑ Slater LB. Malarial birds: modeling infectious human disease in animals. In: Bull Hist Med. 2005; 79 (2): 261-94. PMID 15965289 .
- ↑ Sam R. Telford, Jr .: A Contribution to the Systematics of the Reptilian Malaria Parasites, Family Plasmodiidae (Apicomplexa: Haemospororina). In: Bulletin of the Florida State Museum, Biological Sciences 1988; 34 (2): 67-98.
- ^ Prudêncio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. In: Nat Rev Microbiol . 2006 4 (11): 849-856. PMID 17041632 .
- ↑ Beier JC. Malaria parasite development in mosquitoes. In: Annu Rev Entomol. 1998. 43: 519-43. PMID 9444756 .
- ^ Matuschewski K. Getting infectious: Formation and maturation of Plasmodium sporozoites in the Anopheles vector. In: Cell Microbiol. 2006 8 (10): 1547-1556. PMID 16984410 .
- ↑ Ferreira MU, da Silva Nunes M, Wunderlich G. Antigenic Diversity and Immune Evasion by Malaria Parasites In: Clin. Diagn. Lab. Immuno. 2004, 11 (6) pp. 987-995. PMID 15539495 .
- ↑ Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. "Antimalarial drug discovery. Efficacy models for compound screening" In: Nat Rev Drug Discov . 2004 3 (6): 509-520. PMID 15173840 .
- ↑ Winzeler EA. Malaria research in the post-genomic era. In: Nature 2008 455: 751-756. PMID 18843360 .
- ^ Adl SM, Simpson AG, Farmer MA, Andersen RA, Anderson OR, Barta JR, Bowser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup O, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MF. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. In: J Eukaryot Microbiol. 2005 Vol. 52 (5): 399-451. PMID 16248873 .
- ^ Kuo CH, Wares JP, Kissinger JC. The Apicomplexan whole-genome phylogeny: an analysis of incongruence among gene trees. In: Mol Biol Evol. 2008 Dec; 25 (12): 2689-98. PMID 18820254 .
- ^ Levine ND. Progress in taxonomy of the Apicomplexan protozoa. In: J Protozool. 1988 Nov; 35 (4): 518-20. PMID 3143826 /.
- ↑ a b c d Martinsen ES, Perkins SL, Schall JJ. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. In: Mol Phylogenet Evol. 2008 Apr; 47 (1): 261-73. PMID 18248741 .
- ↑ Martinsen ES, Waite JL, Schall JJ. Morphologically defined subgenera of Plasmodium from avian hosts: test of monophyly by phylogenetic analysis of two mitochondrial genes. In: Parasitology. 2007 Apr; 134 (Pt 4): 483-90. PMID 17147839 .
literature
- Percy Cyril Claude Garnham: Malaria Parasites and other Haemosporidia. Oxford: Blackwell Scientific Publications, 1966. ISBN 0-632-01770-8 .
Web links
- Zilversmit, Martine, and Susan Perkins. 2008. Plasmodium . Malaria parasites. Version 05 May 2008. In The Tree of Life Web Project, http://tolweb.org/