Fluoroquinolone antibiotic
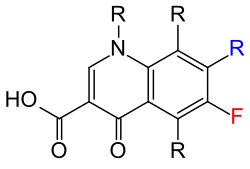
The blue marked residue R is almost always a piperazinyl residue.
The fluorine atom is drawn in red .
Fluoroquinolone antibiotics (also: fluoroquinolones for short ) are a subgroup of the quinolone antibiotics to which they are structurally related. Like these, they act as so-called " gyrase inhibitors " by inhibiting bacterial topoisomerase II (gyrase), an enzyme that plays a role in DNA synthesis. Compared to other gyrase inhibitors, they are characterized by a broader spectrum of activity. Since newer fluoroquinolones are not only active against topoisomerase of type II, but also against other types such as topoisomerase IV , the name gyrase inhibitor has meanwhile become internationally unusual.
Fluoroquinolones are used in both human and veterinary medicine . In 2015, more than four million people with statutory health insurance were prescribed almost 5.9 million medication packs of fluoroquinolones. Since they can cause severe, sometimes irreversible damage to the nervous system and musculoskeletal system , their systemic use in Germany has been restricted since April 8, 2019.
From a chemical point of view, the fluoroquinolones in the backbone are fluorinated at C-6 and also have a piperazine substituent .
Mechanism of action
The bacteria own enzyme gyrase causes the DNA to spiral ( supercoiling ). The fluoroquinolones inhibit the enzyme. The mechanical energy stored in the chromosome of the bacteria decreases and the chromosome length increases. As a result, the bacterial DNA can no longer be replicated correctly . First, the bacterial growth stops (bacteriostatic effect), then the cells die (bactericidal effect). However, an inhibition of DNA replication cannot adequately explain the bactericidal effect of the fluoroquinolones, which is why further mechanisms of action are assumed. Newer representatives of the fluoroquinolones are also effective against bacterial topoisomerase enzymes, which also control the topology of the DNA molecules.
Fluoroquinolones have a very broad spectrum of activity against most gram-negative and gram-positive bacteria , some anaerobes and various streptococci a resistance have. Almost all bacterial infections are considered medical indications , with urinary tract and respiratory tract infections in the foreground. For urinary tract infections, they are recommended in regions with resistance to other antibiotics, especially co- trimoxazole .
Overview of the medicinal substances
The Paul Ehrlich Society for Chemotherapy has proposed a division of fluoroquinolone antibiotics into the following four groups according to pharmacokinetics and spectrum of activity:
- Group I: Oral fluoroquinolones with indication of urinary tract infection
- Group II: Systemically applicable fluoroquinolones with broad indication
- Group III: Fluoroquinolones with improved effectiveness against gram-positive and atypical pathogens
- Group IV: Fluoroquinolones with improved effectiveness against gram-positive and atypical pathogens as well as against anaerobes .
The substances of higher groups are generally also newer products.
Fluoroquinolones used in human medicine
- Enoxacin (group II)
- Norfloxacin (Group I)
- Ciprofloxacin (Group II)
- Ofloxacin (Group II)
- Levofloxacin (Group III)
- Moxifloxacin (Group IV)
- Nadifloxacin
- Lomefloxacin
- Delafloxacin
As of 2019, only the following five are approved in Germany: Norfloxacin, Ciprofloxacin, Ofloxacin, Levofloxacin and Moxifloxacin.
The table below provides an overview of the market withdrawals for human medical fluoroquinolones.
| year | Non-proprietary name | Reason for withdrawal |
|---|---|---|
| 1992 | Temafloxacin | Liver and kidney toxicity, HUS, anaphylaxis, anemia |
| 1993 | Lomefloxacin | Phototoxicity, liver toxicity, CNS ADRs |
| 1998 | Rosoxacin | Marketing decision |
| 1999 | Trovafloxacin | Liver failure, liver toxicity |
| 1999 | Grepafloxacin | Cardiotoxicity |
| 2000 | Pefloxacin | Marketing decision, but also: phototoxicity, tendinitis |
| 2001 | Clinafloxacin | Phototoxicity, influence on sugar metabolism |
| 2001 | Sparfloxacin | Phototoxicity, CNS ADRs |
| 2004 | Fleroxacin | Phototoxicity, CNS ADRs |
| 2004 | Gatifloxacin | Influence on the sugar metabolism |
| 2016 | Enoxacin | Interactions |
Fluoroquinolones used in veterinary medicine
Side effects
During fluoroquinolone treatment, 4-10% of patients experience adverse effects. Some of these are class-specific. According to recent manufacturer information, the incidence of side effects is 25–30%. This increase is explained by the intensification of clinical studies to assess the potential for side effects of the newer fluoroquinolones. The most common side effects are gastrointestinal disorders such as nausea and diarrhea, and central nervous disorders. Psychiatric disturbances with suicidality , blood sugar decompensation in diabetics and tendinitis and ruptures, such as an Achilles tendon rupture, are rarer .
The decrease in tendon strength can occur with all gyrase inhibitors, even after short-term use. The frequency with levofloxacin may be increased. The elderly and those taking corticosteroids are at greater risk. This is explained by an increased expression of matrix metalloproteinases , which reduce the strength of the tendons. Fluoroquinolones can remove the cofactor iron through iron chelation of the α-ketoglutarate -dependent dioxygenase . This probably interferes with collagen maturation . The authors suggest this as a cause of the fluoroquinolone-induced kidney damage and tendinopathy (tendon damage). There is also evidence of direct cytotoxic and antiproliferative (growth-inhibiting) effects as well as damage to the mitochondrial DNA . Histologically, necrosis has been documented in connection with fluoroquinolone-related tendon and kidney damage . The frequency of tendon damage is given in a document from the drug commission of the German medical profession as 1: 227 for ciprofloxacin and 1: 104 for ofloxacin / levofloxacin.
Since all fluoroquinolones have a phototoxic potential, exposure to sunlight or UV light should be avoided. A suspected increased risk of retinal detachment could not be verified in a large Danish study. However, two subsequently published large studies from Taiwan and France could statistically prove an increased risk of rhegmatogenic and exudative retinal detachment when taking fluoroquinolones.
The ingestion of fluoroquinolones can lead to the development of peripheral neuropathy , which can occur a few days after the start of therapy, both orally and intravenously, and then persist for a year or more. The problem here is that this side effect is often not taken seriously enough by doctors and the switch to another antibiotic is therefore delayed.
Retrospective clinical studies associate fluoroquinolone intake with a more than 2-fold increase in risk of aortic aneurysm and aortic dissection . The results of a meta-analysis show that the risk of fluoroquinolone-related aortic damage is increased by at least a factor of 2, with 618 fluoroquinolone prescriptions accounting for aortic damage in the risk group of over 65s. Experimental data suggest that fluoroquinolone-induced aortic dissections are based on necroptosis (in vivo) or apoptosis of aortic smooth muscle cells (in vitro) and destruction of the extracellular matrix of the aortic wall and can lead to fatal aortic ruptures if atherosclerosis is also present . The Federal Institute for Drugs and Medical Devices has on 26 October 2018 due to the increased risk for aortic aneurysms and dissections in a Red Hand Letter ordered the inclusion of a mention in the product information texts of all systemic and inhaled applied fluoroquinolones and new on 8 April 2019 Application restrictions published.
Fluoroquinolone-Associated Disability
In 2015, the FDA first summarized the side effects of fluoroquinolones into a defined disease under the name Fluoroquinolone-Associated Disability (FQAD) . Quinolones are now approved for the treatment of severe infections in children and adolescents and in patients with cystic fibrosis. They should be used with caution, since animal experiments have indicated toxic effects on articular cartilage and the epiphyseal plate .
According to the FDA, the serious side effects of fluoroquinolones outweigh the benefits of treating patients with sinusitis , otitis media , bronchitis, and uncomplicated urinary tract infections for which alternative antibiotics are available. In patients with these diseases, fluoroquinolones should therefore only be used as the last resort after all alternative antibiotics have failed. The safety review showed that systemically applied fluoroquinolones (in the form of tablets and capsules or intravenously ) can lead to disability and potentially permanent severe chronic side effects, several of which can occur at the same time. These side effects include: a. Tendons , muscles , joints , nerves and the central nervous system . Examples are the occurrence of tendinitis , tendopathy , tendon rupture , effects on the central nervous system and peripheral neuropathy . They can lead to permanent impairment in some of the patients. As a result, on July 26, 2016, the American FDA ordered a corresponding update of the package inserts . Of all antibiotics , fluoroquinolones cause the most permanent disability.
In November 2016, fluoroquinolone victims, reported on by Bayerischer Rundfunk and Der Spiegel , created and submitted an online petition to the German Bundestag for co-signature. In the petition, they demand, among other things, a warning symbol on the drug packs and the use of fluoroquinolones only in life-threatening situations.
In February 2017, the Federal Institute for Drugs and Medical Devices ( BfArM ) initiated a European risk assessment process for the serious side effects of fluoroquinolones and quinolones, which can lead to severe impairment of quality of life and possibly permanent impairment. In October 2018, the recommended Pharmacovigilance Committee for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) of the European Medicines Agency for the revaluation of the risk-benefit ratio Limitations. In the case of certain mild to moderate infections, these agents should no longer be used if possible, and in certain patient groups (e.g. elderly patients, patients with renal impairment, patients after organ transplantation or patients under systemic corticosteroid therapy) only with caution.
Class-specific side effects
Representatives of groups III and IV can lengthen the QT interval in the ECG and thus trigger the QT syndrome with ventricular arrhythmias. For moxifloxacin and norfloxacin, hepatotoxic effects resulting in death have been described extremely rarely (only 8 cases in more than 50 million patients). Itching and headache have also been described as side effects . In animal experiments, cartilage damage occurred in growing animals (dogs) .
The Federal Institute for Drugs and Medical Devices has restricted the indication for the antibiotic levofloxacin on the recommendation of the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) . The CHMP had classified levofloxacin as a reserve antibiotic in May 2012 . The approved indications are acute bacterial sinusitis , acute exacerbation of chronic bronchitis , community-acquired pneumonia and complicated skin and soft tissue infections. In future, however, their use must be strictly limited to situations in which "other antibiotics that are usually recommended for the initial treatment of the relevant infections are not considered to be indicated".
Due to their effect not only on bacterial but also on human DNA gyrase, some gyrase inhibitors - such as fleroxacin, gatifloxacin, grepafloxacin , sparfloxacin and trovafloxacin - had to be withdrawn from the market, mainly because of severe toxic complications and intolerance reactions, some of which resulted in death.
history
As the first fluorinated quinolone, norfloxacin was patented in 1979 by the pharmaceutical manufacturer Kyorin Seiyaku Kabushiki Kaisha and approved in Switzerland in 1983. Norfloxacin had good bioavailability, a longer half-life and, above all, affects a wider range of pathogens than nalidixic acid . In the year 1981 by the company Bayer then ciprofloxacin developed and patented 1983rd Levofloxacin was developed by Daiichi Seiyaku and came onto the market in Japan in 1993 and in Germany in 1998.
In 1986, US researchers observed that, in contrast to non-fluorinated quinolones, C-6-fluorinated quinolones have an antibacterial inhibitory effect that is up to 126 times stronger and that the fluorine atom at position 6 binds to the gyrase complex up to 17 times and is up to 70 times stronger - times stronger cell penetration, whereas other substituents introduced at position 6 favor a very weak inhibitory effect. In 1996 the SOS Chromotest showed that C-6-fluorinated quinolones are genotoxic, whereas the non-fluorinated quinolone nalidixic acid is not genotoxic. Between 1988 and 2003, over 350 million patients worldwide were treated with ciprofloxacin, over 250 million with levofloxacin and over 13 million with moxifloxacin.
Web links
- Does triclosan make bacteria resistant? At scinexx - also says the "quinolones" have this possible effect. Since the 2nd figure shows the fluoroquinolone skeleton, these are obviously meant.
Individual evidence
- ↑ a b G. Füllgraf, Björn Lemmer , Kay Brune (eds.): Pharmakotherapie: Klinische Pharmakologie. 13th edition. Springer, 2006, ISBN 3-540-34180-3 , p. 132.
- ↑ AOK Institute criticizes prescription rates for fluoroquinolones. (No longer available online.) AOK Scientific Institute , May 2, 2017, archived from the original on July 12, 2017 ; Retrieved July 18, 2017 .
- ↑ Risk assessment procedure of the Federal Institute for Drug Safety. Retrieved March 27, 2020 .
- ↑ David C. Hooper: Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones . In: Clinical Infectious Diseases . tape 32 , Supplement 1, March 15, 2001, p. S9-S15 , doi : 10.1086 / 319370 .
- ↑ Karl Drlica, Muhammad Malik, Robert J. Kerns, Xilin Zhao: quinolone-Mediated Bacterial Death . In: Antimicrobial Agents and Chemotherapy . tape 52 , no. 2 , February 1, 2008, p. 385-392 , doi : 10.1128 / aac.01617-06 , PMID 17724149 .
- ↑ V. Rafalsky, I. Andreeva, E. Rjabkova: women Quinolones for uncomplicated acute cystitis in . In: Cochrane Database Syst Rev . tape 3 , 2006, p. CD003597 , doi : 10.1002 / 14651858.CD003597.pub2 , PMID 16856014 (English).
- ↑ bfarm.de Serious and long-lasting side effects in the areas of muscles, joints and nervous system , April 8, 2019.
- ↑ a b Gyrase inhibitor: Restrictions on use . In: Information service of the Hessen Association of Statutory Health Insurance Physicians (Hrsg.): KVH current pharmacotherapy . tape 14 , no. 1 . Berlin 2009, p. 30 ( dgn.de [PDF; 2.0 MB ]).
- ↑ KBV medication catalog 2018 , accessed on May 28, 2020.
- ↑ Peter Ball: Efficacy and safety of levofloxacin in the context of other contemporary fluoroquinolones: a review . In: Current Therapeutic Research, Clinical and Experimental . tape 64 , no. 9 , 2003, p. 646-661 , doi : 10.1016 / j.curtheres.2003.11.003 , PMID 24944413 , PMC 4053061 (free full text).
- ↑ Drug Ordinance in Practice 2008; 35 (1), pp. 14-17 ( akdae.de. PDF; 442 kB).
- ↑ RM Harrell: Fluoroquinolone-Induced Tendinopathy: What Do We Know? In: South Med. J. Vol. 92, No. 6, Jun 1999, pp. 622-625.
- ↑ a b R. Stahlmann, H. Lode: Side effects of the newer fluoroquinolones. ( Memento from August 1, 2014 in the Internet Archive ) (PDF; 82 kB). In: Chemotherapy Journal 1998. 7 (3), pp. 107-116.
- ↑ H. Vyas, G. Krishnaswamy: Images in clinical medicine. Quinolone-associated rupture of the Achilles' tendon. In: The New England Journal of Medicine . Volume 357, number 20, November 2007, p. 2067, doi: 10.1056 / NEJMicm061227 . PMID 18003963 .
- ^ S. Badal, YF Her, LJ Maher: Non-antibiotic effects of fluoroquinolones in mammalian cells. In: The Journal of biological chemistry. (Electronic publication before printing) July 2015, doi: 10.1074 / jbc.M115.671222 . PMID 26205818 .
- ↑ Nasim Zargar Baboldashti, Raewyn C. Poulsen, Sarah L. Franklin, Mark S. Thompson, Philippa A. Hulley: Platelet-rich plasma protects tenocytes from adverse side effects of dexamethasone and ciprofloxacin . In: The American Journal of Sports Medicine . tape 39 , no. 9 , September 2011, p. 1929-1935 , doi : 10.1177 / 0363546511407283 , PMID 21632978 .
- ↑ RJ Williams, E. Attia, TL Wickiewicz, JA Hannafin: The effect of ciprofloxacin on tendon, paratenon, and capsular fibroblast metabolism . In: The American Journal of Sports Medicine . tape 28 , no. 3 , May 2000, pp. 364-369 , doi : 10.1177 / 03635465000280031401 , PMID 10843129 .
- Jump up ↑ JW Lawrence, DC Claire, V. Weissig, TC Rowe: Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells . In: Molecular Pharmacology . tape 50 , no. 5 , November 1996, pp. 1178-1188 , PMID 8913349 .
- ↑ K. Bernard-Beaubois, C. Hecquet, G. Hayem, P. Rat, M. Adolphe: In vitro study of cytotoxicity of quinolones on rabbit tenocytes . In: Cell Biology and Toxicology . tape 14 , no. 4 , August 1998, pp. 283-292 , PMID 9733283 .
- ↑ Rafał Kozieł, Krzysztof Zabłocki, Jerzy Duszyński: Calcium Signals Are Affected by Ciprofloxacin as a Consequence of Reduction of Mitochondrial DNA Content in Jurkat Cells . In: Antimicrobial Agents and Chemotherapy . tape 50 , no. 5 , May 1, 2006, pp. 1664-1671 , doi : 10.1128 / aac.50.5.1664-1671.2006 , PMID 16641433 .
- ^ W. Petersen, H. Laprell: The "insidious" rupture of the achilles tendon after ciprofloxacin induced tendinopathy. A case report . In: The trauma surgeon . tape 101 , no. 9 , September 1, 1998, pp. 731-734 , doi : 10.1007 / s001130050330 .
- Jump up ↑ Jc Le Huec, T. Schaeverbeke, D. Chauveaux, J. Rivel, J. Dehais: Epicondylitis after treatment with fluoroquinolone antibiotics . In: The Journal of Bone and Joint Surgery. British volume . 77-B, no. 2 , March 1, 1995, p. 293-295 , doi : 10.1302 / 0301-620x.77b2.7706350 .
- ↑ AJ Dichiara, M. Atkinson, Z. Goodman, KE Sherman: Ciprofloxacin-induced acute cholestatic liver injury and associated renal failure. Case report and review . In: Minerva Gastroenterologica E Dietologica . tape 54 , no. 3 , September 2008, p. 307-315 , PMID 18614979 .
- ^ Nada N. Al-Shawi, Possible Histological Changes Induced by Therapeutic Doses of Ciprofloxacin in Liver and Kidney of Juvenile Rats . In: Pharmacologia . tape 3 , no. 9 , p. 477-480 , doi : 10.5567 / pharmacologia.2012.477.480 .
- ↑ Fluoroquinolones: inflammation and rupture of the Achilles tendon. (PDF; 183 kB) Medicines Commission of the German Medical Association, accessed on July 18, 2017 .
- ↑ Farzin Forooghian: Oral fluoroquinolones and the risk of retinal detachment. In: JAMA. 307, 2012, p. 1414, doi: 10.1001 / jama.2012.383 .
- ↑ Björn Pasternak, Henrik Svanström, Mads Melbye, Anders Hviid: Association Between Oral Fluoroquinolone Use and Retinal Detachment. In: JAMA. 310, 2013, p. 2184, doi: 10.1001 / jama.2013.280500 .
- ↑ Shu-Chen Kuo, Yung-Tai Chen, Yi-Tzu Lee, Nai-Wen Fan, Shih-Jen Chen: Association Between Recent Use of Fluoroquinolones and Rhegmatogenous Retinal Detachment: A Population-Based Cohort Study . In: Clinical Infectious Diseases . tape 58 , no. 2 , January 15, 2014, p. 197-203 , doi : 10.1093 / cid / cit708 .
- ↑ Fanny Raguideau, Magali Lemaitre, Rosemary Dray-Spira, Mahmoud Zureik: Association Between Oral Fluoroquinolone Use and Retinal Detachment . In: JAMA Ophthalmology . tape 134 , no. 4 , April 1, 2016, doi : 10.1001 / jamaophthalmol.2015.6205 .
- ↑ FDA Drug Safety Communication: FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection. August 15, 2013 ( fda.gov ).
- ↑ Permanent nerve damage from fluoroquinolones . In: Deutsches Ärzteblatt . August 16, 2013 ( aerzteblatt.de ).
- ↑ Nick Daneman, Hong Lu, Donald A. Redelmeier: Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study . In: BMJ Open . tape 5 , no. 11 , November 1, 2015, p. e010077 , doi : 10.1136 / bmjopen-2015-010077 , PMID 26582407 .
- ↑ Chien-Chang Lee, Meng-tse Gabriel Lee, Yueh-Sheng Chen, Shih-Hao Lee, Yih-Sharng Chen: Risk of Aortic Dissection and Aortic Aneurysm in Patients Taking Oral Fluoroquinolone . In: JAMA Internal Medicine . tape 175 , no. 11 , November 1, 2015, p. 1839–1847 , doi : 10.1001 / jamainternmed.2015.5389 .
- ↑ Sonal Singh, Amit Nautiyal: Aortic Dissection and Aortic Aneurysms Associated with fluoroquinolones: A Systematic Review and Meta-Analysis . In: The American Journal of Medicine . tape 130 , no. December 12 , 2017, p. 1449-1457.e9 , doi : 10.1016 / j.amjmed.2017.06.029 .
- ^ Scott A. LeMaire, Lin Zhang, Wei Luo, Pingping Ren, Chris Guardado: Abstract 15910: Ciprofloxacin Increases Susceptibility to Aortic Dissection and Rupture in Mice . In: Circulation . tape 136 , Suppl 1, November 14, 2017, p. A15910 ( circ.ahajournals.org [accessed February 21, 2018]).
- ↑ Rote-Hand-Brief on systemic and inhalative fluoroquinolones: Risk of aortic aneurysms and aortic dissections. In: bfarm.de. Federal Institute for Drugs and Medical Devices, October 26, 2018, accessed on October 26, 2018 .
- ↑ Federal Institute for Drugs and Medical Devices (BfArM): Warning notices on antibiotics containing fluoroquinolones: Rote-Hand-Brief informs about serious and long-lasting side effects as well as new restrictions on use. Press release 2/19. Retrieved April 8, 2019 .
- ↑ Martin Whling: Clinical Pharmacology. Thieme, 2005, p. 528.
- ↑ a b FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. ( fda.gov ), May 12, 2016.
- ↑ Information on side effects of fluoroquinolone preparations available in the USA ( fda.gov ).
- ↑ FDA: Fluoroquinolone Safety Labeling Changes. (PDF) In: FDA. FDA, April 2017, accessed January 19, 2019 .
- ↑ Veronika Hackenbroch: Medicine: Two tablets of pain . In: Der Spiegel . No. 7 , 2017, p. 104-105 ( online ).
- ^ Pharmaceuticals - Decision making on fluoroquinolone antibiotics - Online petition. Retrieved April 18, 2017 .
- ^ Moritz Pompl, Carola Brand, Bayerischer Rundfunk: Side effects of drugs: bureaucracy endangers health. (No longer available online.) April 4, 2017, archived from the original on May 20, 2017 ; Retrieved April 18, 2017 .
- ↑ Those affected by fluoroquinolone side effects: Petition to the Bundestag in November 2016. (No longer available online.) November 2016, archived from the original on January 17, 2018 ; Retrieved July 19, 2017 .
- ↑ BfArM - Press - Fluoroquinolones and quinolones: BfArM initiates European risk assessment process. Retrieved April 18, 2017 .
- ↑ Quinolone- and fluoroquinolone-containing medicinal products. EMA, February 10, 2017.
- ↑ Fluoroquinolones: Severe and long-lasting side effects affecting muscles, joints and nervous system. October 5, 2018, accessed October 23, 2018 .
- ↑ D. Adam, K.-F. Bodmann, W. Elies, C. Lebert, KG Naber, K. Simons, A. Pross: Oral antibiotics in clinic and practice: Practice-oriented recommendations for antibiotic therapy of mild to moderate bacterial infections in adults in outpatient and inpatient settings. Springer, 2009, ISBN 978-3-642-00521-3 , p. 24.
- ↑ Levofloxacin: Indication restrictions due to severe side effects . In: Deutsches Ärzteblatt . September 4, 2012 ( aerzteblatt.de ).
- ↑ Wolfgang Forth, Franz Hofmann, Ulrich Förstermann: General and special pharmacology and toxicology. 9th edition. Elsevier, Urban & Fischer Verlag, 2008, ISBN 978-3-437-44490-6 , p. 831.
- ↑ Patent US4146719 - Piperazinyl derivatives of quinoline carboxylic acids. In: Google.com/Patents. Retrieved February 6, 2019 .
- ↑ quinolones. In: PharmaWiki.ch. Retrieved February 6, 2019 .
- ↑ Patent DE3142854 : 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-piperazino-quinoline-3-carboxylic acids, process for their preparation and antibacterial agents containing them. Registered on October 9, 1981 , published on May 11, 1983 , applicant: Bayer, inventor: Klaus Grohe, Hans-Joachim Zeiler, Karl Georg Metzger.
- ↑ Patent DE3033157 : 7-Amino-1-cyclopropyl-4-oxo-1,4-dihydro-naphthyridine-3-carboxylic acids, process for their preparation and antibacterial agents containing them. Registered on September 3, 1980 , published April 1, 1982 , applicant: Bayer, inventor: Klaus Grohe, Hans-Joachim Zeiler, Karl Georg Metzger.
- ↑ Walter Sneader: Drug Discovery. A history . John Wiley & Sons, 2005, ISBN 978-0-470-01552-0 ( limited preview in Google Book Search).
- ^ History of Daiichi - About Us. In: DaiichiSankyo.com . Retrieved February 25, 2017 .
- ↑ John M. Domagala, Lori Doyle Hanna, Carl L. Heifetz, Marland P. Hutt, Thomas F. Mich, Joseph P. Sanchez, Marjorie Solomon: New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. In: Journal of Medicinal Chemistry. 29, 1986, p. 394, doi : 10.1021 / jm00153a015 .
- ↑ L. Majtánová, V. Majtán: Quinolone effects in the SOS chromotest and the synthesis of biomacromolecules . In: Folia Microbiologica . tape 41 , no. 3 , 1996, PMID 9449772 .
- ^ Peter Ball: Efficacy and safety of levofloxacin in the context of other contemporary fluoroquinolones: A review . In: Current Therapeutic Research, Clinical and Experimental . tape 64 , no. 9 , 2003, PMID 24944413 .