Aortic dissection
| Classification according to ICD-10 | |
|---|---|
| I71.0 | Aortic Dissection (Any Section) |
| ICD-10 online (WHO version 2019) | |
In medicine, aortic dissection or aneurysm dissecans aortae refers to the splitting of the wall layers of the main artery ( aorta ), usually caused by a tear in the inner vascular wall ( tunica intima ) with subsequent bleeding between the layers. It usually causes sudden, violent pain and is immediately life-threatening because it can lead to the main artery rupturing (aortic rupture) and to acute circulatory disorders in various organs. While it usually ended fatally 50 years ago, the majority of those affected survive today. This is mainly due to an operation of the most dangerous forms, which was initiated as quickly as possible . Immediate diagnosis is therefore of crucial importance for this disease.
Emergence
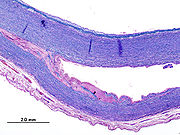
The vascular wall of the aorta, viewed from the outside in, consists of the three layers adventitia ( tunica adventitia ), media ( tunica media ) and intima ( tunica intima ). If the elastic or muscular parts of the media are weakened, bleeding there can expand more or less unhindered and lead to a splitting ( dissection ) of the vascular wall between the intima and adventitia.
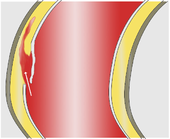
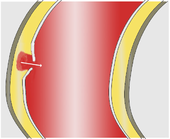
Two different mechanisms are discussed as immediate triggers of the dissection. Most often the first thing to do is to tear the intima, which gives the bloodstream access to the media. (Through this opening engl. : Entry ) presses the arterial blood pressure , the blood between the intima and adventitia where it usually continues to expand in the longitudinal direction of the vessel and an artificial space ( "false lumen creates").
Less often, bleeding into the media seems to be the primary event leading to an initially small bruise ( hematoma ). It comes from the supply vessels ( vasa vasorum , cf. vessel ) of the aorta and in many cases only provides secondary access to the vascular lumen when the pressure of the bruise tears the intima. Some of these hematomas are confined to the vessel wall and remain unconnected to the bloodstream in the aorta ( intramural hematoma).
The extent of the dissection depends largely on the blood pressure as the driving force and the resistance of the media. It can be a few millimeters or the entire length of the aorta, extending into the pelvic arteries and also into side branches such as the carotid arteries and the renal arteries .
Risk factors
The most common predisposing factors are a structural weakness of the media (so-called media degeneration) and arteriosclerosis .
The media degeneration initially leads to an expansion of the ascending aorta and is caused in the majority of cases (80%) by an inadequately treated hypertension disease. Congenital diseases associated with connective tissue changes ( Marfan syndrome , Ehlers-Danlos syndrome , bicuspid aortic valve ) are rarer. The cause here is a cystic median necrosis that is above average for age .
The two factors age and blood pressure are also of great importance for the development of arteriosclerosis. Among other things, it leads to flat wall deposits ( plaques ) on the intima, which tend to tear (plaque rupture), which can lead to sometimes deep bleeding craters . These craters, also known as ulcers , can be the gateway for a dissection.
Rare causes of aortic dissection are coarctation of the aorta and inflammatory diseases of the arteries ( arteritis , see vasculitis ) with involvement of the aorta. The observed frequency of dissections after ingestion of cocaine or crack, as well as in connection with pregnancy, remains unexplained . About half of all dissections in women under the age of 40 occur in the last trimester of pregnancy or shortly after delivery. Since these patients often have Marfan's syndrome or high blood pressure ( hypertension ), the importance of pregnancy as a cause of dissection is rather doubted.
External influences are rare as a cause of aortic dissection. They lead to circumscribed tears, bruises or, in the event of strong violence, to a rupture of the aorta. Injuries can also be caused by medical interventions ( iatrogenic ), such as during a catheter examination or heart surgery, especially after surgical replacement of the aortic valve.
Up to 18% of patients with aortic dissection have had heart surgery at some point before.
Undesired drug effects represent a further risk. For example, fluoroquinolones can possibly promote or trigger an aortic dissection. Retrospective clinical studies associate fluoroquinolone intake with a more than 2-fold increase in risk of aortic aneurysm and aortic dissection. Experimental data suggest that fluoroquinolone-induced aortic dissections are based on necroptosis (in vivo) or apoptosis of aortic smooth muscle cells (in vitro) and destruction of the extracellular matrix of the aortic wall and can lead to fatal aortic ruptures if atherosclerosis is also present .
pathology
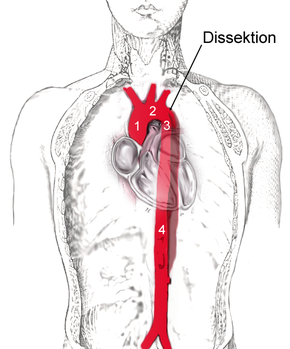
The aortic dissection is created to 80% above and 65% a few centimeters above the aortic valve of the aorta (the rising portion of the ascending aorta ), and 20% immediately after the origin of the left subclavian artery ( subclavian vein ), in the descending portion of the aorta ( aortic descendens ). Furthermore, the aortic arch is affected with 10% or the abdominal artery ( aorta abdominalis ) with 5%.
Pathologically , in the majority of patients, the picture of the classic aortic dissection with a dissection membrane and a tear in the intima can be demonstrated in the sense of entry . In around 10–17% of autopsies , however, there is no such entry , so that one speaks of an intramural hematoma (bruise in the vessel wall).
The intima is often thickened in the course of the dissection, the media thin, with damage to its elastic and muscular parts. In the acute stage, the adventitia is often widened and thinned like a balloon due to the increased pressure, while in chronic stages it appears to be thickened by connective tissue.
Cystic median necrosis (including the type of idiopathic median necrosis Erdheim-Gsell ) is histologically evident in up to 25% of patients ; these findings are more common in patients under the age of 50 than in older people.
More rarely, a crater-shaped ulcer is found on the aortic wall in the area of atherosclerotic wall deposits, which has "eaten" its way through the wall layers and either caused the vessel to burst (aortic rupture) or to a dissection. Such a penetrating atherosclerotic ulcer is more common in elderly patients with hypertension and in smokers.
Epidemiology
Epidemiological data suggest three new cases per 100,000 population ( incidence ) each year . A study from 2017 shows an incidence of up to 11.9 aortic dissections per 100,000 inhabitants. For 1998 the Federal Statistical Office calculated 4.5 deaths from aortic dissection per 100,000 inhabitants. In people over 65 years of age, the dissection, together with the aortic aneurysm, ranks 13th in the cause of death statistics as "aortic disease".
Men get sick twice to three times as often as women. Aortic dissections are most common in 50 to 70 year olds, but patients with congenital beneficial factors are often affected in early adulthood.
For unknown reasons, aortic dissections tend to occur during the winter months. Of the almost 1000 patients recorded in the International Registry of Acute Aortic Dissection (IRAD) up to 2005 , 28.4% suffered the dissection in winter, only 19.9% in summer. It is also unclear why most dissections occur in the morning between 6:00 and 12:00.
Forms of thoracic aortic dissections

|
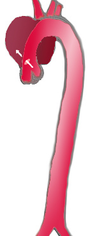
|

|
|
| frequency | 60% | 10-15% | 25-30% |
| Type | DeBakey I | DeBakey II | DeBakey III |
| Stanford A | Stanford B | ||
| Proximal | Distal | ||
| Tab. 1: Classifications of aortic dissection according to DeBakey and PO Daily | |||
With a view to the risk to the patient and the therapeutic consequences, a strict distinction has been made between aortic dissections with and without involvement of the ascending aorta. The classifications in use today are based on this criterion.
In 1965, the American surgeon DeBakey proposed a division into three categories (type DeBakey I to III). In type I, both the ascending aorta and the descending aorta are affected, in type II only the ascending aorta and in type III only the descending aorta.
In order to be able to deduce the risk to the patient and the most important therapeutic steps directly from the classification, this classification was simplified in 1970 by Daily and employees of Stanford University . They combined the types DeBakey I and DeBakey II and only differentiated whether the "entry" is in the area of the ascending aorta (Stanford A type or proximal dissection) or distal to the left subclavian artery (Stanford B type or distal dissection). This classification has become the most widely accepted today.
It is still controversial how the intramural hematoma (IMH) and the penetrating ulcer, considered by many to be a “preliminary stage” or “close relative” of aortic dissection, and the penetrating ulcer are to be classified. The IMH is often viewed and referred to as a dissection. There is also consensus in western countries to treat it like a dissection. In Asian countries there is a more strict separation between the two and a more conservative therapy is advocated for IMH, as spontaneous healing occurs more frequently.
| class 1 | Classic dissection with entry and membrane | ||
| 2nd grade | Medial splitting with intramural bleeding | ||
| Class 3 | Intimar tear with a discreet bulge | ||
| Grade 4 | Aortic ulcer after plaque rupture | ||
| Class 5 | Iatrogenic or traumatic dissection | ||
| Tab. 2: Extended classification (including ESC 2001) | |||
A classification proposed in 1989 and adopted by a working group of the European Society of Cardiology (ESC) in 2001 also includes changes in the aorta that are not dissections in the classical sense, but are similar to or precede it. Although this classification has not yet established itself in clinical routine, it is certainly a common consideration of these diseases of the aorta, which are similar in many respects. The increasingly common term "acute aortic syndrome" encompasses aortic dissection, intramural hematoma, aortic ulcer and rupture of an aortic aneurysm.
A dissection is called acute if its symptoms have not persisted for more than 14 days. Otherwise it is called a chronic dissection.
Clinical picture
The clinical picture varies greatly depending on the affected aortic segment and the involvement of side branches. The spectrum ranges from the absence of any symptoms and signs of illness to sudden cardiac death due to an early rupture of the aorta or the occlusion of a coronary artery .
If the dissection begins proximally , i.e. in the ascending aorta, complications from the heart are in the foreground. A rupture in the pericardium leads to bloody pericardial effusion with threatening cardiac tamponade . A widening of the aortic root or even the progression of the dissection into the cusps of the aortic valve can result in its inability to close ( aortic valve insufficiency ). The expansion of the dissection into one of the coronary arteries often results in a circulatory disorder of the heart muscle and even a heart attack .
Circulatory disorders of the arms and head ( aortic arch syndrome ) indicate involvement of the aortic arch. In the descending aorta, dissection can lead to circulatory disorders in the intestines , kidneys , legs, and spinal cord .
Symptoms
Typical of the aortic dissection and the main symptom is violent and sudden pain, which is described by 80–96% of all those affected. It is often experienced as tearing or stabbing and usually begins immediately with maximum intensity. Usually it is so strong that the event is assessed by patients, relatives and doctors as highly acute and threatening. It is not uncommon for patients to hunch over in pain or pass out. With proximal dissections the pain begins in the chest area, with distal dissections it often begins in the back between the shoulder blades. Almost every fifth patient feels the pain as wandering, which is explained by the progression of the dissection along the course of the aorta. However, the dissection can also be painless ("silent" or asymptomatic), so that it is only discovered by chance in people who are symptom-free.
Any other symptoms are less common, they are caused by the possible complications:
- Shortness of breath and shock symptoms with cardiac involvement,
- Pain in the affected extremities with circulatory disorders of the arms or legs,
- Symptoms of a stroke when the arteries supplying the brain are involved,
- Abdominal or flank pain with circulatory disorders of the intestines or kidneys,
- Symptoms of paralysis when there is insufficient blood supply to the spinal cord ,
- severe, sudden pain in the jaw
Clinical signs
There are no typical clinical signs of the aortic dissection itself, these only arise from the possible consequences. In the case of greater blood loss, the signs of shock such as increased pulse rate , drop in blood pressure and clouding of consciousness are impressive . Circulatory disorders in the extremities lead to a weakening or loss of pulse. A reduced blood supply to the intestine can cause a mesenteric infarction with corresponding signs, circulatory disorders of the brain result in signs of a stroke. Aortic valve insufficiency can be identified during auscultation by the typical diastolic heart murmur , while pericardial effusion can be identified by a weakening of the heart's sounds and a paradoxical pulse ( pulsus paradoxus ).
Technical findings
Decisive examination procedures for suspected dissection are the X-ray of the thoracic organs , the ultrasound examination ( sonography ) especially in the form of the transesophageal echocardiography (TEE), the computed tomography (CT) and the magnetic resonance tomography (MRT), in individual cases also the angiography .
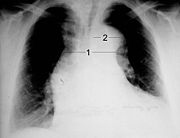
The dissection itself cannot be seen on a normal x-ray, but indirect indications in the form of a widening or double contour of the mediastinum or the aorta may be visible. Mediastinal widening can be detected in about 63% of patients with type A dissection and about 56% of those with type B dissection. Altogether, if a dissection is suspected, some abnormal findings are found in 60–90% of the radiographs, but the absence of such a finding does not rule out a dissection.

|

|
| Transesophageal Echocardiography (TEE) |
TEA with color Doppler colored true lumen |
|
Cross section through the ascending aorta 1 dissection membrane - 2 aortic valve |
|
With the usual ultrasound examination of the abdomen ( abdominal sonography), the sections of the aorta ( aorta abdominalis ) located in the abdomen can usually be displayed and assessed more or less well. In addition, the first two to five centimeters of the aorta can often be assessed moderately well with the normal ultrasound examination of the heart ( echocardiography ). Overall, however, the diagnostic accuracy of the simple ultrasound method for the detection of aortic diseases is only moderate because the quality of the image through the chest and abdominal wall is often poor and parts of the ascending aorta and the aortic arch and the sections of the descending aorta in the chest cannot be assessed ( Sensitivity 59–85%, specificity 63–96%).
The TEE, on the other hand, allows a good representation of the aortic sections near the heart and the descending aorta in the chest cavity. Due to the high spatial resolution, an existing dissection membrane can almost always be recognized, and with the help of Doppler technology , the true lumen can be reliably differentiated from the false lumen. In many cases the entry and a possibly existing re-entry are visible. Aortic valve insufficiency can be diagnosed just as precisely as pericardial effusion . In contrast, changes to the aortic arch, to the vessels supplying the brain there and to the abdominal aorta are often inadequately represented in the TEE; these regions are also referred to as "blind spots" in the TEE. The sensitivity for the detection of aortic diseases is 88–99%, the specificity 95–98%.

|

|
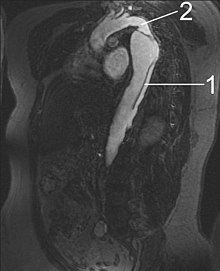
| Computed tomography (CT) | Legend |
|
Aortic dissection type Stanford A 1 ascending aorta, true lumen - 2 false lumens - 3 pulmonary artery 4 descending aorta - 5 thoracic vertebrae |
|
A CT performed with a contrast agent allows a comprehensive and exact representation of the entire aorta and can reliably display both the dissection itself and its spatial relationship to the side branches of the aorta and any bleeding in the area. A possible involvement of the aortic valve is not recognizable, however, the aortic sections near the valve are more difficult to assess due to artifacts caused by the beating heart with older CT machines. With these devices, a sensitivity of 83–94% and a specificity of 87–100% have been determined in several studies; modern spiral CTs achieve a sensitivity of more than 95% on average.
The representation in the MRI , for which no iodine-containing contrast agent is required and which also allows a reliable assessment of the aortic valve, is similarly precise and comprehensive . The MRI achieves the best sensitivity and specificity for the diagnosis of aortic dissection with almost 100% each.
With angiography, the side branches of the aorta and any aortic valve insufficiency that may be present are easy to assess; the dissection leads to different contrast staining of the true and false lumen and to an unusual spread of the contrast medium. At around 70%, the sensitivity of angiography is lower than with the aforementioned methods, mainly due to the inadequate detection of dissections without connection to the true lumen of the aorta. In contrast, the specificity of the order of 95% is high.
Other examination techniques do not help, and laboratory tests have so far been of little use in aortic dissection. Anemia can be detected in the case of high blood loss , an increase in D-dimers in the case of thrombosis of the wrong lumen, and in larger dissections also the number of leukocytes and C-reactive protein , but these changes are too ambiguous ( unspecific ) to contribute to the differential diagnosis . In the future, metalloproteinases and myosin heavy chains could play a role in laboratory medical diagnostics of aortic dissection.
Course of disease
The further course of an aortic dissection is variable and cannot be predicted with any certainty. The high pressure in the wrong lumen can tear the outer layer of the vessel (adventitia) within minutes and lead to fatal bleeding to death. Further progression of the dissection along the course of the vessel is also possible. This can happen “several times” in bursts and, as the dissection extends to the kidney, intestinal or leg arteries, it can lead to individual episodes of further organ involvement.
If the blood flow in the wrong lumen is directed back into the true lumen through one or more further openings in the intima ( re-entry ), the risk of rupture is initially lower. The two lumens that are perfused and separated by the dissection membrane can persist for years. This situation is typical of the uncomplicated chronic Stanford B dissections. "Pseudo-healing" by thrombosing the wrong lumen is also possible.
diagnosis
Aortic dissection is one of the most pressing emergencies in cardiology and cardiac surgery ; Their diagnostics are demanding because they require the use of complex and not immediately available procedures under time pressure. The high mortality of one to two percent per hour in the case of type A dissection in the acute phase makes it necessary to clarify the suspected diagnosis immediately using transesophageal echocardiography (TEE), CT or MRI.
Initial suspicion
Any sudden severe pain in the back, chest or abdomen for which no other plausible explanation can be found must also suggest an aortic dissection or an aortic aneurysm. In particular, if there are additional indications such as increased blood pressure (49% of patients with aortic dissection), pulse or blood pressure differences (31%), a diastolic heart murmur (28%) or focal neurological deficits (17%), the suspicion of aortic dissection must be expressed and clarified unless proven otherwise.
Investigation process
Any suspicion of an aortic dissection that has arisen must be confirmed or ruled out as quickly as possible with the help of one of the appropriate imaging methods . The choice of procedure depends on its availability, the experience of the examiner involved, and the patient's condition.
- Transthoracic and transesophageal echocardiography are available in almost every hospital and can also be performed on unstable patients in the intensive care unit or in the operating room. The risks are minimal and there is no radiation exposure. The accuracy of echocardiography is very dependent on the experience of the examiner.
- Computed tomography is also widespread, but always requires the patient to be transported to the X-ray department, where monitoring options may be limited. The CT is associated with a not inconsiderable radiation exposure, which, however, has to be accepted, at least for non-pregnant women, in view of the far-reaching consequences of a possible misdiagnosis. The necessary administration of contrast medium leads to an additional risk of an allergic reaction or renal dysfunction in about one out of a hundred examinations.
- Magnetic resonance imaging is the most advantageous in terms of diagnostic accuracy, but only available around the clock in a few hospitals. In addition, the ability to monitor patients is often severely limited by the design of the usual devices, so that MRI is only conditionally suitable for unstable patients. There are hardly any additional risks; any contrast media used are well tolerated, provided that the kidneys are functioning normally. The examination of patients with metallic foreign bodies or cardiac pacemakers is not without problems because of the intense magnetic fields .
In practice, the first diagnostic procedure used in over 1000 patients in the IRAD register was CT in 61%, echocardiography (TTE and TEE) in 33%, angiography in 4% and MRI in 2%. Quite often, the diagnosis was supplemented by a second procedure, on average 1.8 procedures were used. The second method used most frequently was echocardiography (56%), followed by CT (18%), angiography (17%) and MRI (9%).
Differential diagnosis
In view of the main symptom, other diseases with similarly intense and sudden onset pain are also considered in the case of an aortic dissection. A myocardial infarction is often based on the electrocardiogram are deferred (ECG). In the differential diagnosis of chest pain, acute aortic syndrome is the second most common life-threatening disease after acute coronary syndrome and before pulmonary embolism . For every 80 to 300 patients with acute coronary syndrome, one patient with acute aortic syndrome comes to the emergency room.
Biliary or renal colic can often be differentiated with the help of an ultrasound examination. Occasionally, increased pancreatic lipase helps further in the context of laboratory diagnostics because it suggests an inflammation of the pancreas .
Often, however, the above-mentioned procedures do not yield any conclusive findings, so that a transesophageal echocardiography or computed tomography are inevitable because of the critical time factor. Only when thus dissection was sufficiently excluded certain further studies are useful, then from pulmonary embolism of a herniated disc or a "pinched nerve" to the pleurisy can uncover a variety of other causes of pain.
forecast
Until the 1960s, the prognosis for patients with an aortic dissection was catastrophic. Acute type A dissections were found in various studies with a mortality rate of 30–70% within 24 hours and 80–95% in the first week. Since three out of four deaths occurred in the first two weeks, the risk of chronic dissections was estimated to be lower, but hardly a patient survived the first year.
| Period | Without surgery | With surgery | |
|---|---|---|---|
| In 24 hours | 20% | 10% | |
| In a week | 40% | 13% | |
| In a month | 50% | 20% | |
| Tab. 3: Lethality with type A dissection | |||
Today these patients have a better prognosis. The data of more than 1100 patients compiled in the international register IRAD show a still considerable but clearly lower mortality for type A dissections (cf. Tab. 3). These figures also make it clear that the operation, which is common today, makes a decisive contribution to this improvement in prognosis.
The prognosis is better for type B dissections that are limited to the descending aorta. One- and two-year survival rates of 80–90% were found for them with purely drug therapy. The IRAD data for this group of patients shows a 30-day mortality rate of around 20% for operated patients and around ten percent for those who did not have to undergo surgery.
therapy
In the case of an acute dissection, in addition to adequate pain therapy, the focus is first on avoiding complications. With close monitoring of the circulatory parameters ( monitoring ), beta blockers and possibly sodium nitroprusside are used to lower blood pressure to systolic values of around 110 mmHg in high blood pressure ; as a rule, highly effective opiates are used to combat pain .
Otherwise, the basic principles of therapy for dissections in the aorta near the heart (Stanford A type) differ fundamentally from those in the descending aorta (Stanford B type) because of their different prognoses.
Proximal Dissection (Type A)
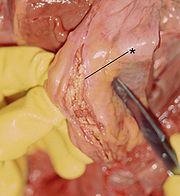
In the case of an acute type A dissection, it is important to avert the risk of rupture as quickly as possible. Standard therapy is the immediate surgical replacement of the ascending aorta with a vascular prosthesis . Occasionally, the aortic valve can also be reconstructed as part of an emergency procedure. As a rule, however, the aortic valve is removed in the case of dissection of the aortic sections near the valve and patients with a congenital connective tissue disease (e.g. Marfan syndrome) and a prosthesis with an integrated valve prosthesis ( valve- bearing conduit or composite prosthesis ), to which the coronary arteries are re-used Be "connected". The 30-day post-operative mortality for type A dissection is 15–30%.
Even with chronic type A dissections, surgical correction is almost always carried out. However, the time factor is of secondary importance here so that the intervention does not have to be carried out in an emergency. In addition, since the wall layers, especially of the false lumen, are usually thicker than in an acute dissection, the sutures are technically easier to create and are more durable.
The prostheses are usually made of woven dacron fabric, which is coated with collagen for primary blood tightness. A replacement of the large vessels with Goretex prostheses is rather untypical.
Distal dissection (type B)
With uncomplicated type B dissections, the 30-day mortality rate after surgical therapy is around 25% higher than with pure drug therapy with less than ten percent. For this reason, surgery is only performed in the event of life-threatening complications, such as a rapidly increasing diameter of the aorta or other signs of an impending or already existing rupture.
The treatment of other complications is now mainly interventional , i.e. H. with the help of percutaneously inserted catheters . In the case of percutaneous intimal membrane fenestration (PFA), the dissection membrane can be fixed with one or more stents or “windowed” through an artificial re-entry using the catheter in order to reduce the risk of rupture. Sealed side branches can often be reopened, dilated and fixed with a stent.
Stents in the thoracic artery ( aorta thoracica ) are called TEVAR (thoracic endovascular aortic repair), in the abdominal artery ( aorta abdominalis ) EVAR (endovascular aortic repair).
In uncomplicated type B dissections, an additional endovascular intervention (stenting) does not offer any advantage over an optimal drug setting alone; one study showed rather a (statistically insignificant) trend towards increased 2-year mortality after stent implantation.
history
The first description of an aortic dissection dates from the time of Galen in the second century AD, a mention by Vesalius comes from 1557 . Morgagni reported an aortic dissection into the pericardial space in 1761:
- A man ... was gripped by pain in his right arm and shortly afterwards his left arm ... afterwards a tumor appeared on the upper part of the sternum. ... He was directed to think seriously and piously of his departure from this mortal life that was imminent and inevitable. (Translation to)
The term dissection probably comes from René Laënnec , who first wrote in 1819 about "aneurysm dissecans". Shennan laid the foundation for today's understanding of aortic dissection with a publication in 1934.
Surgical treatment of aortic dissection was first proposed in 1935 by Gurin et al. a. Tried by windowing the dissection, the patient did not survive. In 1955 Shaw treated with surgical fenestration, and this patient also died. In the same year, however, an American team of surgeons led by DeBakey reported the first successful operation for an acute aortic dissection. In 2015, the German Heart Center Berlin developed the unique concept of the "aortic telephone" for better preclinical care of aortic emergencies. A central emergency number was set up for Berlin and Brandenburg, through which the aortic emergency is coordinated and supported with appropriate technical support. This resulted in a significantly better care time and an increase in the number of cases.
Veterinary medicine
Aortic dissections have also been described in cattle , dogs and cats as well as at least one gorilla and one African ostrich . However, they are almost exclusively diagnosed after the death of the animal ( post-mortem ). The causes are also in veterinary medicine u. a. Elevated blood pressure and congenital connective tissue diseases similar to Marfan's syndrome have been identified. Because of the anatomical and pathophysiological similarities, artificially produced dissections in dogs and pigs have also been used in research into surgical and interventional therapy methods.
In horses Aortenabrisse are more common, not only during exercise, but also in the pasture. The case of the world-class show jumping horse Hickstead became known .
literature
- EM Isselbacher: Aortic Dissection . In: DP Zipes u. a. (Ed.): Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th edition. WB Saunders, Philadelphia 2004, ISBN 1-4160-0014-3 .
- Horst Rieger, Andreas L. Strauss, Werner Schoop (Eds.): Clinical Angiology. 1st edition. Springer, Berlin 1998, ISBN 3-540-50899-6 .
- R. Erbel et al. a .: Diagnosis and Management of Aortic Dissection . (PDF, 622 kB) In: Eur Heart J . 2001, 22 (18), pp. 1642-1681, PMID 11511117 (English); Comprehensive and commented recommendations from the ESC Task Force
- Y von Kodolitsch u. a .: The acute aortic syndrome . In: Dtsch Arztebl. 2003, 100, pp. A 326-333.
- E. Weigang et al. a .: Management of patients with aortic dissection . In: Dtsch Arztebl. 2008, 105, pp. A 639-645.
- Reinhard Larsen: Anesthesia and intensive medicine in cardiac, thoracic and vascular surgery. (1st edition 1986) 5th edition. Springer, Berlin / Heidelberg / New York et al. 1999, ISBN 3-540-65024-5 , pp. 405-425; here: p. 406 f.
Web links
- Ultrasound film sequences aortic dissection and aortic ulcer ( MOV ) Steinkopff-Verlag
- Case description with several film sequences Echocardiography, TEE and CT ( Flash ) Journal for Cardiology , 2005, Krause & Pachernegg Verlag
Individual evidence
- ↑ Erdmann (Ed.): Clinical Cardiology . 8th edition. Springer, Heidelberg 2011, ISBN 978-3-642-16480-4 , pp. 453 f .
- ↑ CA Nienaber et al. a .: Diagnosis and management of aortic dissection - orientation between recommendations and register data. In: Cardiology up2date. 2005, 1, pp. 63-76.
- ↑ Nick Daneman, Hong Lu, Donald A. Redelmeier: Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study . In: BMJ Open . tape 5 , no. 11 , November 1, 2015, p. e010077 , doi : 10.1136 / bmjopen-2015-010077 , PMID 26582407 .
- ↑ Chien-Chang Lee, Meng-tse Gabriel Lee, Yueh-Sheng Chen, Shih-Hao Lee, Yih-Sharng Chen: Risk of Aortic Dissection and Aortic Aneurysm in Patients Taking Oral Fluoroquinolone . In: JAMA Internal Medicine . tape 175 , no. 11 , November 1, 2015, p. 1839–1847 , doi : 10.1001 / jamainternmed.2015.5389 .
- ^ Scott A. LeMaire, Lin Zhang, Wei Luo, Pingping Ren, Chris Guardado: Abstract 15910: Ciprofloxacin Increases Susceptibility to Aortic Dissection and Rupture in Mice . In: Circulation . tape 136 , Suppl 1, November 14, 2017, p. A15910 ( circ.ahajournals.org [accessed February 21, 2018]).
- ^ J. Ostermeyer: Heart and vessels close to the heart. In: Rudolf Häring, Hans Zilch (Hrsg.): Textbook surgery. 2nd, revised edition. De Gruyter, Berlin / New York 1988, ISBN 3-11-011280-9 , pp. 342-384, here: p. 377.
- ↑ SD Kurz, V. Falk, J. Kempfert, M. Gieb, TM Ruschinski: Insight into the incidence of acute aortic dissection in the German region of Berlin and Brandenburg . In: International Journal of Cardiology . tape 241 , August 2017, ISSN 0167-5273 , p. 326–329 , doi : 10.1016 / j.ijcard.2017.05.024 ( elsevier.com [accessed on May 8, 2018]).
- ↑ H. Eggebrecht u. a .: Echocardiographic evaluation of the patient with acute chest pain in the emergency room. In: Intensivmed. 2006, 43, pp. 64-77.
- ↑ RH Mehta et al. a .: Predicting death in patients with acute type A aortic dissection. In: Circulation. 2002, 105, pp. 200-206, PMID 11790701 .
- ↑ CA Nienaber et al. a .: Randomized Comparison of Strategies for Type B Aortic Dissection: The INvestigation of STEnt Grafts in Aortic Dissection (INSTEAD) Trial. In: Circulation. 2009, 120 (25), pp. 2519-2528, PMID 19996018 .
- ↑ M. Klompas: Does This Patient Have an Acute Thoracic Aortic Dissection? In: JAMA. 2002, 287, pp. 2262-2272, PMID 11980527 .
- ^ T. Shennan: Dissecting Aneurysms. His Majesty's Stationery Office, London 1934. Medical Research Clinical Special Report Series No. 193
- ↑ Berlin concept 'aortic telephone': When the arterial wall ruptures. In: Doctors newspaper. Retrieved May 8, 2018 .
- ↑ NRW state stud stallion Beijing is dead , Dominique Wehrmann, St. Georg , March 28, 2018
- ^ Aortic Rupture , Marcia King, The Horse, April 1, 1999
- ↑ Hickstead: Aortic avulsion confirmed . St. Georg November 10, 2011