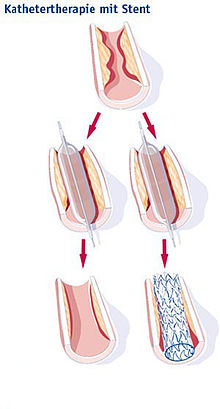
Stent

A stent (German vascular support ) is a medical implant for holding open vessels or hollow organs. It is mostly a spiral wire prosthesis in the form of a tube made of metal or synthetic fibers with auxetic or mechanical properties for vasodilation.
On the one hand, stents are used in blood vessels , especially the coronary arteries , in order to prevent renewed occlusion after their expansion ( PTCA ); such treatment is known as stent angioplasty . On the other hand, stents are used in cancer treatment to keep open airways ( windpipe ), biliary tract or esophagus constrictions caused by malignant tumors after an expansion.
Origin of the word
There are several derivations of the word stent:
- According to the Oxford English Dictionary , the word “stenting” in English has been used to describe the stiffening or strengthening of clothing for centuries.
- In medical history, the word stent seems to go back to the English dentist Charles Stent (1807–1885). Stent invented a material in 1856 that was used in oral and facial surgery to make impressions of teeth and jaws. In 1916 Jan F. Esser , a Dutch plastic surgeon, first referred to the material invented by Charles Stent in writing. Esser was quoted in 1920 in a textbook Plastic Surgery of the Face by the English military doctor HD Gillies, who first used the word stent as a noun for the shape made from stent material.
- The shape of the stent used today was initially called “wall stent”.
Expandable stents were developed in the late 1970s by Julio Palmaz in Texas, who then received a patent in 1988 and worked with Johnson & Johnson.
Medical aspects
Blood vessel stents, which are mainly used in the coronary arteries, can close again. On the one hand, this can happen a short time after the stent has been inserted, since the stent, being a foreign material, stimulates the local blood coagulation, so that a thrombus can form in the stent. Such an in-stent thrombosis is often fulminantly fatal. It is therefore important to inhibit the function of the blood platelets ( thrombocytes ) in particular after the stent has been inserted . Platelet aggregation inhibition with acetylsalicylic acid has established itself as the basic therapy . In the first time, an additional inhibition takes place via another point of attack by tirofiban intravenously during the stent insertion or later with clopidogrel , ticagrelor or prasugrel , which can be taken as tablets like acetylsalicylic acid. This double inhibition of platelet function is known as dual inhibition of platelet aggregation.
In addition, the stent may slowly close over time due to the formation of new connective tissue. Such restenosis should be prevented by drug-releasing stents (DES, see below), which inhibit the formation of new tissue. Since it takes longer until these stents are covered with endothelium and no longer stimulate blood clotting, the dual platelet aggregation inhibition must take longer.
In the meantime, acute coronary angiography with expansion of the narrowed coronary arteries and stent insertion is the therapy of choice for acute myocardial infarction if a cath lab can be reached promptly. Risks when stents are inserted into the coronary arteries include injury to the vessel with bleeding, acute thrombosis with infarction of the tissue supplied by the vessel, and the unintentional occlusion of side branches of the vessel by the stent.
In ophthalmology, stents are used as part of microinvasive glaucoma surgery (MIGS). The small implants improve the outflow of aqueous humor from the eye and thus lower the intraocular pressure. The iStent, for example, creates a connection between the anterior chamber of the eye and the natural drainage path, Schlemm's canal, through the trabecular meshwork.
materials
Initially, the coronary stents were made of L512 stainless steel.
Drug- eluting stents
A further development is the use of vascular stents that are filled with active substances, e.g. B. glucocorticoids, cytostatics, immunomodulators or antiproliferatives are coated.
A drug eluting stent (DES) releases small amounts of drugs that inhibit the formation of new cells. Two active ingredients have established themselves in the treatment with drug-eluting stents: the immunosuppressant sirolimus and the cancer therapeutic paclitaxel . Such stents have been used in Germany since 2002, alongside the conventional uncoated stent ( bare metal stent / BMS), primarily for the treatment of coronary heart disease .
Cordis was the first to introduce the drug-eluting stent Cypher . By now, almost all major companies have a coated stent on the market, including Boston Scientific , Medtronic , Terumo, and Abbott . The main differences between the stents are the drugs and polymers used. A cobalt-chromium alloy (L605) or a cobalt-nickel alloy is usually used for the coated steel framework.
The current data situation for comparing the different stent types is not clear and the discussion about this is not over. For example, studies continue to compare drug-eluting stents with uncoated stents, stents with bypass, and drug-eluting stents. A distinction is also made between different patient groups, such as patients with acute myocardial infarction or diabetes mellitus .
Meta-analyzes show no significant difference between drug-eluting stents and uncoated stents with regard to the mortality rate of patients, although an increased stent thrombosis rate was observed in the long-term with paclitaxel stents. It is currently recommended that drug-eluting stents be used preferably when there is an increased risk of restenosis (as in diabetics), but cautiously when there is an increased risk of stent thrombosis.
Drug eluting stents should not be used if there is a possibility of prolonged administration of clopidogrel, e.g. B. is not given because of an upcoming surgical treatment or if it is to be expected that the patient will not comply with the medication.
A meta-analysis by the Technical University of Munich, which summarizes all study results available worldwide on drug-eluting stents and thus takes into account data from a total of 3669 patients, seems to show a superiority of the sirolimus-releasing stent compared to paclitaxel: Accordingly, the risk of restenosis with sirolimus is half as much big as with paclitaxel.
Bioabsorbable stents
background
The use of drug-eluting stents made of metals or alloys has some disadvantages. Over time, thromboses can occur, the vessel is no longer so flexible, revascularization is hindered and the evaluation of computed tomography images is made more difficult.
Therefore, different types of bioabsorbable stents are currently being developed. The basic idea is to only support the vessel as long as this is clinically necessary. After the support phase of a few weeks to a few months, these stents dissolve in the patient's body and, in contrast to the known permanent stents, allow the blood vessel to move freely again (vasomotion).
Researchers found that bio-stents increase the risk of heart attacks. Originally it was hoped that the bio-stent would cause irritation of the vessel wall less frequently, since no foreign body would remain in the vessel with the implant. The latest studies on stents show, however, that bio-stents lead to significantly more complications, especially more than a year after implantation. As a result, Abbott took the product off the market worldwide in mid-July 2017.
materials
Traditional biocompatible metals such as tantalum , titanium or chromium degrade too slowly because a passivation layer forms on them in the body. In addition, the breakdown products cannot be metabolized. However, metals that play a role in metabolism are biocompatible. The two most suitable candidates are therefore alloys of magnesium and iron . Pure magnesium degrades too quickly. The main focus of research on metallic stents is on the control of corrosion and passivation of the alloys in the respective application area. Good results have been achieved with biodegradable metallic glass . Alloys made from magnesium and from rare earth metals are also suitable , since rare earths have low cytotoxicity .
Instead of metals, degradable polymers can also be used for stents. A typical material is polylactate (PLA). The research focuses primarily on achieving the good properties of conventional permanent metal stents with the polymeric materials. In particular, the mechanics and biocompatibility represent a hurdle for polymer approaches.
Radioactive stents
The radioactivity of activated stents prevents the excessive cell growth of the inner skin of the blood vessel and prevents restenosis. To avoid unwanted side effects such as To prevent, for example, the leaching of drugs or radionuclides into the bloodstream, techniques such as ion implantation or activation of the basic material ( neutron activation ) are used. Nevertheless, radiation protection problems arise in practice.
The first radioactive stent was produced in 1992 at the Karlsruhe Research Center (FZK, today KIT); it was a stent made of steel, the alloying elements of which were activated.
The most important nuclides with regard to the stent coating are palladium -103 and phosphorus -32. The radioactive isotope of phosphorus was introduced into the base material of the stent for the first time using ion implantation . A homogeneous distribution over the entire stent and good adhesion to the base material were achieved. The effectiveness of this product has been confirmed in clinical studies.
In order to provide a stent with palladium , a gold layer is first applied to the stent by electroplating . This serves as an adhesion promoter for the palladium, which is also deposited galvanically on the stent. The palladium is then covered with a final layer of gold to prevent it from being washed out. The undesired low-energy gamma radiation of the palladium is also absorbed in this way .
Radioactive stents currently play no role in medical practice. Correspondingly, since 2007 (in Germany) no additional remuneration has been paid .
Case numbers and costs
On average in the EU, 191 stents are implanted per 100,000 inhabitants per year; in Germany there are 624 (3.3 times as many). According to the OECD, there is no country in the world that uses more stents than Germany. A study that appeared in the journal The Lancet in early November 2017 found that a stent offers few advantages over any stent - for patients without a heart attack. Over 500,000 stents are used in German clinics every year. The health insurances reimburse 3182 euros for a stent, in difficult cases 4135 euros.
Web links
Individual evidence
- ^ In: Frankfurter Allgemeine Zeitung . January 6, 2010, p. N1.
- ↑ Peter Reuter: Springer Lexicon Medicine. Springer, Berlin a. a. 2004, ISBN 3-540-20412-1 (Lemma Stent).
- ↑ Thomas Michel: Treatment of Myocardial Ischemia. 1941. In: Laurence L. Brunton, John S. Lazo, Keith L. Parker: Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th edition. McGraw-Hill, New York 2006, ISBN 0-07-142280-3 , p. 842.
- ↑ Guidelines for myocardial infarction therapy : Acute coronary syndrome with persistent ST segment elevation (STEMI). (PDF; 2.1 MB)
- ^ Grace Richter, Anne Coleman: Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol 2016: 10 189-206
- ↑ Ronald D. Gerste: Micro-invasive glaucoma surgery - a suitable addition to small incision cataract surgery. Ophthalmic surgery 2015; 27: 103
- ↑ In: The Lancet . 2007 Sep 15; 370 (9591), pp. 937-948.
- ↑ Coronary stents that release medication and balloon catheters coated with medication - Position paper of DGK doi : 10.1007 / s12181-011-0375-6
- ^ In: J Am Coll Cardiol . 2007 Oct 2; 50 (14), pp. 1373-1380.
- ↑ PW Serruys, JA Ormiston, Y. Onuma et al.: A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. In: Lancet. 373, No. 9667, March 14, 2009, pp. 897-910. PMID 19286089 , doi : 10.1016 / S0140-6736 (09) 60325-1
- ↑ JA Ormiston, PW Serruys, E. Regar et al .: A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. In: Lancet. 371, No. 9616, March 15, 2008, pp. 899-907. PMID 18342684 , doi : 10.1016 / S0140-6736 (08) 60415-8
- ↑ Bio-stents fail in practical tests. In: Neue Zürcher Zeitung , November 3, 2017, accessed on November 5, 2017.
- ↑ Biodegradable stents increase the risk of myocardial infarction. In: heilpraxisnet.de, November 4, 2017, accessed on November 4, 2017.
- ↑ [1]
- ↑ sueddeutsche.de February 28, 2018: How a German heart specialist got 200,000 stock options from Singapore