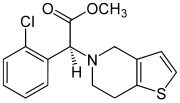
Clopidogrel
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
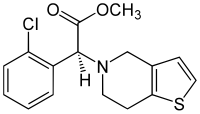
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Clopidogrel | ||||||||||||
| other names | |||||||||||||
| Molecular formula |
Salts:
|
||||||||||||
| Brief description |
|
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| properties | |||||||||||||
| Molar mass | |||||||||||||
| Physical state |
liquid |
||||||||||||
| Melting point |
184 ° C |
||||||||||||
| solubility |
|
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Clopidogrel is a drug that affects hemostasis (blood clotting). The substance works by inhibiting platelet aggregation . Clopidogrel is used for therapy and to prevent blood clots from forming. Such a clot ( thrombus ) develops in the course of the thrombocytic hemostasis and can cause blockages , especially in the arterial part of the vascular system, which lead to a heart attack or stroke or to infarcts in other organs (e.g. mesenteric infarction ). Acetylsalicylic acid (ASA) has a similar but significantly weaker effect to that of clopidogrel, but it acts on platelets via a different mechanism.
Mechanism of action
Clopidogrel is a prodrug , the pharmacologically active metabolite is only formed after absorption through oxidation via cytochrome P450 ( CYP2C19 and other CYP enzymes) and subsequent hydrolysis . Therefore, potent inhibitors of cytochrome P450 can limit the bioactivation of clopidogrel and thus its effectiveness.
The active metabolite of clopidogrel is an inhibitor of the adenosine diphosphate (ADP) receptor of subtype P2Y 12 from the family of inhibitory G i protein-coupled purine receptors ( GPCR ). This receptor plays a central role in glycoprotein IIb / IIIa activation and mediates ADP-induced platelet aggregation on the surface of the thrombocytes (blood platelets) . Clopidogrel therefore acts as an inhibitor of platelet aggregation .
The pharmacologically active metabolite blocks the binding of adenosine diphosphate (ADP) to its platelet receptor (P2Y 12 - receptor ) so that ADP-dependent platelet activation through the glycoprotein IIb / IIIa receptor complex is omitted. The mechanism of action of clopidogrel differs from that of acetylsalicylic acid, which also inhibits platelet aggregation , but by inhibiting the cyclooxygenases COX-1 (and COX-2 ).
Since the blockage of the P2Y 12 receptor is irreversible, the platelets remain impaired throughout their lifespan. After stopping the drug, the full coagulation ability can only be restored with the formation of new platelets in the course of 5 to 7 days.
ADP -induced aggregometry offers one way of assessing the effect of clopidogrel .
synthesis
Clopidogrel is chiral ; In the literature, multi-step synthetic routes for clopidogrel are described. Of the two enantiomers , the ( S ) - (+) - form is used pharmaceutically, with clopidogrel in the form of a salt , e.g. B. hydrogen sulfate , besilate ( benzene sulfonate ) or hydrochloride , is used.
application areas
Clopidogrel is indicated for the prevention of atherothrombotic events:
- as monotherapy after a recent heart attack or in the presence of peripheral arterial occlusive disease (PAD). Due to its side effect profile, the prophylaxis of a stroke can only be used if there is an increased risk of recurrence or if there are other reasons for prescribing it.
- in combination with acetylsalicylic acid
- an acute coronary syndrome without ST segment elevation ( "unstable angina pectoris " or "non-Q-wave myocardial infarction" , see ECG nomenclature ), even if a stent has been inserted into an occluded or narrowed coronary artery , to restore blood flow,
- after a heart attack with ST segment elevation ( STEMI , “ST segment elevation myocardial infarction”) accompanying thrombolysis
- Since the maximum effect can only be expected after 3 to 7 days at a dose of 75 mg, the administration of a loading dose is indicated in acute coronary syndrome. After the usual loading dose of 300 mg or - according to the ISAR-REACT study better - 600 mg of clopidogrel, the effect occurs after two (to six) hours.
Studies
A significantly better efficacy compared to ASA in monotherapy could be seen in studies in the indication “symptomatic PAD ” (CAPRIE study).
As part of a coronary vascular stent insert, both substances are given at the same time. While ASA 100 mg should be administered permanently, i.e. for life after a stent implantation, according to the current state of knowledge, the duration of treatment with clopidogrel 75 mg depends on the one hand on the type of stent implanted (four weeks for pure metal stents, usually six to twelve months) drug-eluting stents), on the other hand after the acuity of the coronary syndrome (nine months for acute coronary syndrome ). The exact duration of this double inhibition of platelet aggregation is controversial and the subject of scientific discussion.
In the context of a stroke, the double inhibition of platelet aggregation does not improve the treatment outcome and leads to more severe bleeding (MATCH study). For this reason, monotherapy with ASA is usually recommended in stroke patients. Clopidogrel monotherapy is only considered in so-called high-risk patients.
Side effects and restrictions on use
Since clopidogrel inhibits blood clotting, the most common undesirable effects are bleeding, for example nosebleeds , stomach or intestinal bleeding , hematomas (bruises), blood in the urine , and in a few cases also bleeding from vessels in the eye, inside the head, in the lungs or joints. In addition, gastrointestinal complaints and occasionally headaches , drowsiness , dizziness and skin rashes can occur. Thrombotic thrombocytopenic purpura (TTP, Moschcowitz syndrome) is probably a very rare side effect . Compared to acetylsalicylic acid, side effects in the gastrointestinal tract are less common with clopidogrel.
Treatment with clopidogrel should only be started after the catheter has been removed after anesthesia procedures close to the spinal cord such as spinal anesthesia or epidural anesthesia . At least seven days should have elapsed since the last dose of clopidogrel before anesthesia near the spinal cord. Likewise, clopidogrel should be discontinued seven days before a planned operation if an effect that inhibits platelet aggregation is temporarily undesirable.
If clopidogrel is overdosed or z. If, for example, the clopidogrel effect is to be canceled immediately because of an emergency operation, the problem is that the platelets in the body are irretrievably inhibited. There is no antidote to counteract the effects of clopidogrel. Since platelets have a lifespan of 7 to 10 days and new platelets are only produced slowly and continuously, it takes several days until sufficiently functional new platelets are formed again. If the platelet function z. B. must be restored immediately because of an emergency operation, foreign blood platelet concentrates must be given. It should be noted that even 6 hours after taking clopidogrel, 50% of the clopidogrel is still present in the blood (half-life) and the new platelets administered are thereby also inhibited.
Pharmacokinetics
- Bioavailability : 50%
- Metabolism : liver
- Half-life : 7–8 hours
- Elimination: 50% kidney , 46% liver
Interaction between clopidogrel and proton pump inhibitors
Clopidogrel is a prodrug which is converted into its active form via the enzyme cytochrome P450 2C19 (an isoenzyme of cytochrome P450 ). Some proton pump inhibitors , such as omeprazole and lansoprazole , are also broken down by this enzyme. Reduced plasma levels of the active clopidogrel metabolite have been found with concomitant treatment with these drugs . There is evidence from studies that this interaction is not clinically relevant.
The cytochrome P450 2C19 is also subject to a pronounced genetic polymorphism . It can either be formed less or more. In the case of “poor metabolizers”, the competition between the proton pump inhibitor and clopidogrel for the CYP2C19 can exacerbate the situation that too little clopidogrel is converted into the active form.
Clopidogrel resistance
Various studies have shown that clopidogrel does not work well enough in 5 to 44% of patients. One reason for this clopidogrel resistance is the frequent loss of function of the gene CYP2C19, whose gene product, cytochrome P450 2C19, is involved in the development of the active ingredient from the ineffective prodrug.
Around 25% of the white American population and around 30% of the Afro-American population carry a CYP2C19 allele with lost or reduced function of the enzyme in the processing of clopidogrel. It is unclear whether an increase in effectiveness could be achieved with a partial resistance through higher doses. Since the beginning of 2013 there has been a test with which the individual activity of the CYP2C19 can be determined in order to determine the possible ineffectiveness of the clopidogrel.
Development and marketing
Clopidogrel was developed jointly by Sanofi-Aventis and Bristol-Myers Squibb as its structural variant after ticlopidine (market entry in 1993) ; it differs from this by a methyl formate side chain. Plavix and Iscover came onto the market for the first time in 1998 as clopidogrel-containing drugs, and were among the first to be approved across the EU via the central approval process introduced in 1995.
After the first approvals for generics containing clopidogrel had already been granted in Germany in May 2008, the first supplier Sanofi-Aventis had tried in vain to prevent their market launch because they had violated the 10- annual document protection (which ended in July 2008) on the dossier of the original product. However, the new preparations had not received the marketing authorization under the drug law through an authorization based on the clinical data of the first supplier, but through the new authorization procedure, although only a comparative study carried out in India with 46 test persons of clopidogrel besilate with clopidogrel hydrogen sulfate was presented. The early market access was explosive against the background that Plavix was the drug with the world's second largest sales. The newly approved preparations were initially around a fifth cheaper than the original preparations.
While Sanofi-Aventis and Bristol-Myers Squibb use clopidogrel in the form of its hydrogen sulfate salt in Iscover and Plavix , respectively, the generics contain other salts (clopidogrel besylate , clopidogrel hydrochloride). This does not infringe the patent rights of the original suppliers. There is no evidence of a difference in effectiveness.
Sanofi-Aventis also developed a product consisting of a fixed combination of 75 mg clopidogrel and 75 or 100 mg acetylsalicylic acid, which enables combined maintenance therapy (see indications) with a single tablet.
Cost-benefit comparison
In 2009, clopidogrel 75 mg was around € 247 per 100 tablets as the original preparation, around 80 times as expensive and as a generic at around € 180 around 60 times as expensive as ASA 100 mg (as a generic around € 3 per 100 tablets).
On behalf of the Federal Joint Committee (G-BA), the Institute for Quality and Efficiency in Health Care (IQWiG ) examined whether this 60 to 80 times higher price than acetylsalicylic acid (ASA) is justified or whether this preparation does not have any superior benefit compared to treatment with ASS owns. In the final report published on June 30, 2006, the institute came to the conclusion: "Compared to treatment with ASA in patients with symptomatic peripheral arterial disease, long-term therapy with clopidogrel (monotherapy) has an additional benefit in terms of reducing the risk of vascular / thromboembolic events. ”However, the report continues, there is no evidence of a reduction in all-cause mortality.
Trade names
Monopreparations
- 75 mg: Plavix (EU, CH), Iscover (EU), Clogombix (A), various generics
- 300 mg: Plavix (EU, CH), Iscover (EU)
Combination drugs
- 75 mg clopidogrel + 75 mg or 100 mg acetylsalicylic acid: DuoPlavin (EU, CH), DuoCover (EU)
Web links
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Clopidogrel
Individual evidence
- ↑ a b c d The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station, NJ, USA, 2006, ISBN 0-911910-00-X , p. 403.
- ↑ DrugBank.
- ↑ Scientific review report for Iscover the European Medicines Agency, October 24 of 2006.
- ↑ Scientific evaluation report for Clopidogrel ratiopharm of the European Medicines Agency, October 16, 2009.
- ↑ Scientific evaluation report for Clopidogrel DURA of the European Medicines Agency, October 14, 2009.
- ↑ Data sheet (±) Clopidogrel hydrogensulfate from Sigma-Aldrich , accessed on May 25, 2011 ( PDF ).
- ↑ S. Murugappa, SP Kunapuli: The role of ADP receptors in platelet function . In: Front. Biosci. tape 11 , no. 1 , 2006, p. 1977-1986 , doi : 10.2741 / 1939 , PMID 16368572 .
- ↑ G. Hollopeter, HM Jantzen, D. Vincent, G. Li, L. England, V. Ramakrishnan, RB Yang, P. Nurden, A. Nurden, D. Julius, PB Conley: Identification of the platelet ADP receptor targeted by antithrombotic drugs . In: Nature . tape 409 , no. 6817 , 2001, p. 202-207 , doi : 10.1038 / 35051599 , PMID 11196645 .
- ^ RA Nicholas: Identification of the P2Y (12) receptor: a novel member of the P2Y family of receptors activated by extracellular nucleotides . In: Mol. Pharmacol. tape 60 , no. 3 , 2001, p. 416-420 , PMID 11502870 ( molpharm.aspetjournals.org ).
- ↑ a b Mutschler, Geisslinger, Kroemer, Schäfer-Korting: Mutschler drug effects. 9th edition. 2008, ISBN 978-3-8047-1952-1 , p. 512.
- ^ Axel Kleemann , Jürgen Engel, Bernhard Kutscher, Dietmar Reichert: Pharmaceutical Substances. Syntheses, Patents and Applications of the most relevant APIs . 5th, compl. revised Edition. Thieme, Stuttgart / New York 2009, ISBN 978-3-13-558405-8 , pp. 526-528 (Original title: Pharmaceutical Active Ingredients. Syntheses, Patents, Applications . The English edition is based on the revised and updated 2nd German edition from 1995, ISBN 3-13-558402-X ).
- ^ S. Yusuf, F. Zhao, SR Mehta, S. Chrolavicius, G. Tognoni, KK Fox: Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. In: The New England Journal of Medicine. 345, No. 7, 2001, pp. 494-502, doi: 10.1056 / NEJMoa010746 . PMID 11519503 .
- ↑ Shamir R. Mehta et al .: Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. In: The Lancet. 358, No. 9281, 2001, pp. 527-533, doi: 10.1016 / S0140-6736 (01) 05701-4 . PMID 11520521 .
- ↑ Marc S. Sabatine et al: Addition of Clopidogrel to Aspirin and Fibrinolytic Therapy for Myocardial Infarction with ST-Segment Elevation. In: New England Journal of Medicine. 352, No. 12, 2005, pp. 1179-1189, doi: 10.1056 / NEJMoa050522 . PMID 15758000 .
- ^ German Society for Cardiology, guidelines .
- ↑ Clopidogrel plus acetylsalicylic acid for acute coronary syndrome ( Memento of the original from March 6, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ Iscover ® product information (PDF; 757 kB), as of September 2009, on the website of the European Medicines Agency .
- ↑ Wiebke Gogarten, Hugo Van Aken: Perioperative thrombosis prophylaxis - platelet aggregation inhibitors - importance for anesthesia In: AINS - anesthesiology · intensive care medicine · emergency medicine · pain therapy. 47, 2012, pp. 242-252, doi: 10.1055 / s-0032-1310414 .
- ↑ Technical information Iscover 75 mg, as of May 2011.
- ↑ Technical information Iscover 75 mg, as of May 2011.
- ↑ DL Bhatt, BL Cryer, CF Contant et al: Clopidogrel with or without Omeprazole in Coronary Artery Disease . In: N Engl J Med . October 2010, doi : 10.1056 / NEJMoa1007964 , PMID 20925534 .
- ↑ Larry Husten: ESC: TIMI study offers reassurance on concominant use of PPIs and antiplatelets . In: CardioBrief. August 31, 2009.
- ↑ M. Gilard et al .: Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. In: J Am Coll Cardiol . 51 (3), Jan 22, 2008, pp. 256-260. PMID 18206732 .
- ↑ New study: A common class of GI medications reduce protection against heart attack in patients taking widely prescribed cardiovascular drug. Medco Health Solutions, November 11, 2008.
- ↑ FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug , Safety Announcement, US Food and Drug Administration, March 12, 2010.
- ↑ JM Lee, S. Park, DJ Shin, D. Choi, CY Shim, YG Ko et al: Relation of genetic polymorphisms in the cytochrome P450 gene with clopidogrel resistance after drug-eluting stent implantation in Koreans. In: Am J Cardiol. 104, 2009, pp. 46-51.
- ^ JE Mobley, SJ Bresee, DC Wortham, RM Craft, CC Snider, RC Carroll: Frequency of nonresponse antiplatelet activity of clopidogrel during pretreatment for cardiac catheterization. In: Am J Cardiol. 93, 2004, pp. 456-458.
- ↑ DJ Angiolillo, A. Fernandez-Ortiz, E. Bernardo, C. Ramírez, C. Barrera-Ramirez, M. Sabaté et al .: Identification of low responders to a 300 mg clopidogrel loading dose in patients undergoing coronary stenting. In: Thromb Res. 115, 2005, pp. 101-108.
- ↑ Kyoung-Jin Park, Hae-Sun Chung, Suk-Ran Kim, Hee-Jin Kim, Ju-Yong Han, Soo-Youn Lee: Clinical, Pharmacokinetic, and Pharmacogenetic Determinants of Clopidogrel Resistance in Korean Patients with Acute Coronary Syndrome. In: Korean J Lab Med. 31, No. 2, 2011, pp. 91-94. PMC 3116006 (free full text).
- ↑ Jeffrey I. Weitz: Antiplatelet, Anticoagulant and Fibrinolytic Drugs. In: Anthony S. Fauci et al. (Ed.): Harrison's Principles of Internal Medicine. 18th edition. New York 2012, p. 990 f.
- ↑ Assessment Report for Clopidogrel Acino (PDF; 502 kB) , European Medicines Agency, 2008.
- ↑ C. Baumgärtel: Generic clopidogrel - The Medicines Agency's Perspective. In: GaBI Journal. Volume 1, Issue 2, 2012, pp. 89-91. (PDF; 151 kB).
- ↑ DuoCover: Product information and public assessment report of the European Medicines Agency, March 31, 2010 (PDF; 740 kB).
- ↑ DuoPlavin: Product information and public assessment report of the European Medicines Agency (PDF; 72 kB), March 31, 2010.
- ↑ Final report on clopidogrel versus ASA in secondary prophylaxis . Pdf IQWiG. Clopidogrel versus acetylsalicylic acid in the secondary prophylaxis of vascular diseases. Final report A04 / 01A. Cologne: Institute for Quality and Efficiency in Health Care (IQWiG), June 2006 . (PDF; 12.5 MB).