Epidural anesthesia
The epidural anesthesia (from Greek περί peri , German , besides, all around ' , dura [mater] , the hard [meningeal]' , and Greek ἀναισθησία , insensibility ',' anesthesia '; abbreviation PDA ; synonymous epidural anesthesia (EDA), from Greek επί epi , German , over, on ' , the German outdated and extradural ) is a form of (epidural) regional anesthesia (see also spinal anesthesia ). It causes the temporary, reversible function inhibition of selected nerve segments, leads to sympathicolysis , numbness, freedom from pain and inhibition of active mobility in the associated body section and, in addition to painless deliveries (here often also neuroaxial analgesia , more rarely also called neuroaxial blockade ) , it also enables otherwise painful delivery medical procedures or pain management for certain causes.
On the history of epidural anesthesia, developments
In 1921, Fidel Pagés described the anestesia metamérica , both in the Revista Española de Cirugía and the Revista de Sanidad Militar , the epidural anesthesia. He drew on the experience of 43 operations carried out. In 1922 he was promoted to Comandante Médico .
Around the mid-1920s, it was the versatile Italian (heart) surgeon Achille Mario Dogliotti (1897–1966) who advocated this technique. In 1941 Robert Andrew Hingson (1913-1996) and Waldo B. Edwards developed the technique of continuous caudal anesthesia with a lying needle. In 1947 it was Manuel Martínez Curbelo (1906–1962) who first described the placement of a lumbar epidural catheter. In Germany, Karl Julius Anselmino and colleagues are named as the first to carry out the method.
A catheter technique that has been established since 1942 and has also proven itself for the lumbar access since around 1949 is usually used. After finding the epidural space, a thin plastic catheter is inserted through the Tuohy needle , which can be left in the epidural space for a few days (and sometimes much longer). This enables a therapy period beyond the actual surgical intervention or a longer-term therapy for chronic pain. Usually, a pump is connected to the catheter, via which a basic amount ( basal rate ) of a local anesthetic, often with the addition of an opioid, is continuously supplied. These pumps also make it possible for patients to give themselves additional doses at the push of a button ( patient controlled epidural analgesia , PCEA) and thus independently achieve pain relief independently of doctors or nursing staff. Overdosing is largely prevented by the pump software , but daily monitoring of the amount of painkillers supplied, as well as the catheter itself and its effect, are essential. These measures make it possible to mobilize patients painlessly again at an early stage after operations and to prevent complications ( pneumonia , thrombosis , shortening of muscles, stiff joints) caused by lack of exercise .
Anatomical basics
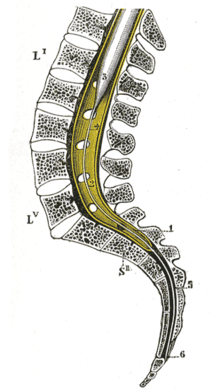
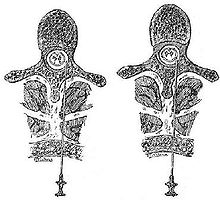
In the area of the spinal cord , the nerve cells and fibers are protected by several layers of connective tissue , the membranes of the spinal cord . From the inside to the outside, these are: the pia mater , a thin layer of supporting cells that lies directly on the spinal cord and also radiates into it, the arachnoid and, as the outer boundary, the dura mater , the hard skin of the spinal cord. The dura mater divides into an inner and an outer sheet; the outer leaf is also the periosteum of the vertebral bodies of the vertebral canal . The so-called epidural space , into which the local anesthetic is injected during epidural anesthesia , lies between the inner and outer sheets of the dura mater .
During the growth of the people is growing spine faster than the spinal cord, so that the spinal cord at the level of the first lumbar vertebra ends, but the associated nerve fibers continue caudally exit the spinal canal (see figure). This must be taken into account when choosing the puncture site, as this is not necessarily at the same height as the site of the operation. In other mammals, the spinal cord extends roughly to the transition between the last lumbar vertebra and the sacrum . Here, epidural anesthesia is usually performed between the sacrum and the first caudal vertebra.
execution
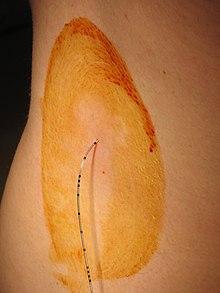
The epidural anesthesia (PDA) is applied while sitting or lying on your side. Ask the patient to relax their shoulders and arch their back. The choice of the height of the puncture site on the patient's spine depends primarily on the location of the operation. After disinfection and local anesthesia of the skin, an epidural needle ( Tuohy needle (see photo) or the Sprotte cannula developed by Günter Sprotte with the Pajunk company and introduced in 1979 ) is inserted into the patient's back between two spinous processes of the spine . The needle penetrates the following structures during insertion: skin - intervertebral ligaments - ligamentum flavum . The so-called "loss-of-resistance" technique is used to identify the epidural space when this needle is advanced. A syringe with liquid is attached to the needle. If the needle is still in front of the epidural space in ligaments of the spine, the injection of liquid is not possible (resistance). While constantly monitoring this injection resistance, the needle is carefully advanced further (usually approx. 4–5 cm deep) until liquid can suddenly be injected without resistance (loss of resistance). This marks the emergence of the needle tip from the ligament structure into the epidural space. A local anesthetic ( e.g. bupivacaine ) injected through the needle into the epidural space now acts on the nerve structures in the spinal canal from outside the dura mater . The Tuohy needle is removed after the injection.
Difference to spinal anesthesia
With spinal anesthesia , a much finer needle is inserted deeper so that the hard meninges ( dura mater ), in contrast to epidural anesthesia, is penetrated. Injected local anesthetic now spreads freely in the cerebrospinal fluid and the spinal cord and nerve fibers in the spinal canal are anesthetized within a few minutes. Compared to epidural anesthesia, significantly smaller amounts of the local anesthetic are required to eliminate the sensation, since the volume of spread is smaller and the diffusion distance is smaller. This also explains the faster onset of action of the spinal anesthesia.
application areas
Epidural anesthesia (PDA) can, depending on the concentration of the local anesthetic used, be used for pain relief and to completely eliminate pain. In major abdominal surgery, orthopedic , gynecological or urological interventions, an epidural catheter is usually put on preoperatively so that it can be used postoperatively for pain therapy . This can reduce the need for other pain relievers (e.g. opioids ) and the incidence of bowel motility disorders.
Another important field of application of the PDA is its use in obstetrics . With low concentrations of local anesthetics, the pain of spontaneous delivery can be reduced considerably. In the case of birth complications and the peridural catheter already in place, a caesarean section can be performed with a higher concentration of the LA while completely eliminating pain . In urgent operations, however, spinal anesthesia is preferred because of the simpler technology and the faster onset of action.
Long-term epidural anesthesia (up to a few months) can be used for palliative treatment of severe chronic pain, for example also with patient-controlled epidural anesthesia . If a longer stay is planned, the epidural catheter is often "tunneled" a few centimeters under the skin from the exit point on the back in order to avoid infection of the epidural space during a longer period of therapy.
Due to the anatomically determined segmental structure and the resulting neural supply, the likely main pain zones can be foreseen with some degree of certainty. Depending on this, the peridural catheter is placed at the appropriate height on the spine. As a guide for the puncture height, the tip of the peridural catheter should be approximately in the middle of the segments to be blocked. The following table can serve as a guide; Upper abdominal interventions are operations on the stomach, pancreas or the transverse colon or transverse intestine , lower abdominal interventions such as urological or gynecological interventions, fracture operations, lower extremities such as knee joint operations.

| Surgical site | Puncture height | Targeted spread of analgesia |
|---|---|---|
| Thoracotomy | Thoracic Th 6–7 | Thoracic Th 2 - 8 |
| thoraco-abdominal surgery (two-cavity surgery) | Th 7-8 and Th 8-9 | Th 4-12 |
| Upper abdominal surgery | Th 8-9 and Th 9-10 | Th 6-12 |
| Abdominal surgery | Th 10-11 and Th 11-12 | Th 8 - Lumbar 2 |
| Surgery on the abdominal aorta | Th 10-11 and Th 11-12 | Th 8-L2 |
| Operations on the lower extremity | Lumbar 3-4 | Th 12 - sacred 1 |
Side effects and complications
- The technical failure or the suboptimal effectiveness of epidural anesthesia are occasionally the cause of increased pain.
- Drop in blood pressure. Blocking the nerves that constrict the vessels ( vasoconstriction ) causes the vessels to widen (vasodilation). The areas that are under the influence of epidural anesthesia feel warm due to the increased blood flow. The vasodilation occasionally leads to a drop in blood pressure, which, however, can usually be avoided by adding fluids (infusion). With thoracic epidural anesthesia in particular, there is a risk of threatening complications due to the elimination of vital compensation mechanisms in CHD patients.
- Injury to the dura mater with a Tuohy needle (0.6–1.3%). This happens when the thick Tuohy needle is advanced too far and punctures the dura mater. Cerebrospinal fluid can now escape through the hole created. This leads to an intense post- puncture headache in 16–86% . Younger patients in particular may be affected here. Such a risk can be significantly reduced by using atraumatic pencil-point needles.
- Accidental total spinal anesthesia . If the dura puncture just described is not noticed by the anesthetist and the entire amount of local anesthetic intended for the epidural space is now injected into the spinal space, this can lead to sharp drops in blood pressure, respiratory paralysis and slowing of the heartbeat up to cardiac arrest. However, every anesthetist should be able to cope with this situation without the patient suffering permanent damage (ventilation, vasopressors, atropine, etc.)
- Injury to the spinal cord. A complication that occurs extremely rarely, but carries the risk of permanent paraplegia.
- Epidural bruise from injury to a vein in the epidural space. Minor bleeding is quite common, but it is self-contained and does not cause clinical symptoms. However, if it bleeds unchecked into the epidural space, the pressure of the resulting effusion can permanently damage the spinal cord. Such a bruise with neurological symptoms occurs in about 1: 150,000 epidural anesthesia; with coagulation disorders there is an increased risk (1: 3000). Only emergency neurosurgical surgery and decompression can now prevent permanent damage.
- Systemic side effects of the local anesthetic used are also possible, for example neurotoxic and cardiotoxic symptoms; allergy
- Meningitis (0.02%)
Requirements for applying an epidural anesthesia
- Consent of the patient
- intact spine ; After surgery on the lumbar spine, for example, epidural anesthesia is sometimes difficult.
- no neurological or psychiatric disorder (addressable patient); epidural anesthesia for a patient with multiple sclerosis, for example, is not a problem. However, it must be clear to the patient that pre-existing neurological symptoms can worsen (but also improve) as part of the planned operation, as this is in the nature of this disease. This is therefore not due to the epidural anesthesia per se.
- intact blood clotting
- Quick value > 50%
- PTT to 50
- Platelets > 80,000 / μl *
- Corresponding time interval to anticoagulant therapy , see detailed table in the next section .
- no infection in the injection area
- no sepsis
- no severe cardiovascular disease (e.g. coronary heart disease applies to the application of epidural anesthesia in the lumbar spine)
- no hypovolemia , no shock
(*) A lack of blood platelets ( thrombocytopenia ) increases the risk of a bleeding complication. An absolute lower limit, up to which spinal anesthesia can be performed, has not been specified by the specialist societies. Rather, the overall coagulation situation must be taken into account. The transfusion of platelet concentrates to increase the platelet count in the blood before spinal anesthesia is recommended from a value of less than 50,000 / μl, so that this can be used as a guide for the lower limit. For epidural anesthesia, where thicker needles are usually used, a transfusion is recommended for less than 80,000 / μl.
Contraindications to epidural anesthesia
- Patient rejection
- Coagulation disorder
- sepsis
- Local infection in the injection area
- Neurological disease (relative contraindication, forensic reasons)
- Bone metastases
- Hypovolemia
- Heart failure
- Allergy to materials or anesthetic
Medicinal anticoagulant therapy
Anticoagulant therapy requires, depending on the drug, a certain time interval to regional anesthesia procedures close to the spinal cord, such as epidural anesthesia. The following table provides an overview of the intervals to be observed between the administration of an anticoagulant before or after the puncture or catheter removal.
| Drug (s) | Before puncture or catheter removal | After puncture or catheter removal |
|---|---|---|
| Unfractionated heparin (UFH) | 4 - 6 h | 1 h |
| Low molecular weight heparins, LMWH prophylaxis | 12 h | 4 h |
| Low molecular weight heparins, LMWH therapy | 24 hours | 4 h |
| Fondaparinux | 36 - 42 h | 6 - 12 h |
| Vitamin K antagonists | INR <1.4 | after catheter removal |
| Clopidogrel | 7 days | after catheter removal |
| Prasugrel | 7-10 days | 6 h |
| Ticlopidine | 10 days | after catheter removal |
| Abciximab | 48 h | 4 h |
| Tirofiban | 8 h | 4 h |
| Prostacyclin (PGI 2 ) | 0.5 h | immediately |
| Dabigatran etexilate | > 34 h | 4 - 6 h |
| Rivaroxaban | 22-26 h | 4 - 6 h |
| Apixaban | 26 - 30 h | 4 - 6 h |
| Ticagrelor | 5 days | 6 h |
| Cilostazol | 42 h | 5 h |
| Dipyridamole plus ASA | 48 h | immediately |
See also
- Epidural cannula
- Spinal anesthesia
- Spinal cannula
- Combined spinal and epidural anesthesia
- Caudal anesthesia
- Lumbar puncture
literature
- Roulhac D. Toledano, Lawrence C. Tsen: Epidural Catheter Design History, Innovations, and Clinical Implications . In: Anesthesiology , V. 121, No 1, July 2014
- J. Antonio Aldrete: Manuel Martinez Curbelo And Continuous Lumbar Epidural Anesthesia . (PDF) In: Bulletin Of Anesthesia History , Volume 22, Number 4, October 2004
- Arkadiusz Praski: The different birth process for patients with PDA and without PDA , Saarland University and State Library, Saarland 2016 DNB 1121581226 (online dissertation Saarland University Saarbrücken 2016, 100 pages, academic supervisor: Erich-Franz Solomayer full text online PDF, free of charge, 100 pages, 1.2 MB).
Web links
- Epidural anesthesia - information at Gesundheitsinformation.de (online offer of the Institute for Quality and Efficiency in Health Care )
- Epidural anesthesia at Familienplanung.de - Information portal of the Federal Center for Health Education (BZgA)
Individual evidence
- ↑ Achille Mario Dogliotti : A new method of regional anesthesia "The peridural segmentar anesthesia" . In: Zentralfl F Chir 1931, 58, pp. 3141-3145
- ^ J. Antonio Aldrete: Manuel Martinez Curbelo And Continuous Lumbar Epidural Anesthesia . (PDF) In: Bulletin Of Anesthesia History , Volume 22, Number 4 October, 2004, pp. 3–8.
- ^ Siegfried Potthoff, Lutwin Beck: On the history of drug and psychosomatic birth relief. In: Lutwin Beck (ed.): On the history of gynecology and obstetrics. On the occasion of the 100th anniversary of the German Society for Gynecology and Obstetrics . Berlin / Heidelberg / New York 1986, pp. 133–141, esp. P. 137. The corresponding article title is: Karl Julius Anselmino, Gerhard Plaskuda, Rudolf Stewens: About a new method of protracted conduction anesthesia of labor pain, the segmental, peridural plomb . In: Klinische Wochenschrift , Volume 27, 1949, Issue 5–6, pp. 104 ff.
- ↑ RA Hingson, JL Southworth: Continuous caudal anesthesia. In: The American Journal of Surgery . 58 (1942), pp. 93ff.
- ↑ A. Doughty: Epidural analgesia in labor: the past. the present and future. In: Proceedings R. Soc. Med. 71 (1978), pp. 879ff.
- ↑ 40 years of Sprotte®. A success story for four decades. In: Anaesthesiology & Intensive Care Medicine. Volume 61, January 2020, spine.
- ↑ Illustration of the innervation and regional anesthesiological possibilities for a woman giving birth ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ^ Hans Walter Striebel: Operative Intensive Care Medicine: Safety in Clinical Practice. Schattauer Verlag, 2007, ISBN 978-3-7945-2480-8 , pp. 22-23.
- ↑ Reinhard Larsen: Anesthesia. Elsevier, Urban & Fischer Verlag, Munich 2011, ISBN 978-3-437-22502-4 , p. 591.
- ↑ Emery Andrew Rovenstine , EM Papper, SE Bradley: Circulatory adjustment during spinal anesthesia in normal man with special reference to the autonomy of arteriolar tone. Anesthesiology 3 (1942), pp. 442ff.
- ↑ W. Zink, BM Graf: Toxicology of local anesthetics, pathomechanisms - clinic - therapy. (PDF; 474 kB) Clinic for Anaesthesiology, Heidelberg University Hospital. In: Anaesthesiologist. (2003) 52, pp. 1102-1123, doi: 10.1007 / s00101-003-0617-5 , published online: November 18, 2003.
- ↑ Szalata Marek: lipid administration in toxic effects of local anesthetics. (PDF; 754 kB) In: Lipid Rescue TM.
- ↑ Regional anesthesia close to the spinal cord and thromboembolism prophylaxis / antithrombotic medication . (PDF) 2nd revised recommendation of the German Society for Anesthesiology and Intensive Care Medicine.
- ↑ Implementation of analgesia and anesthesia procedures in obstetrics 2. Revised recommendations of the German Society for Anesthesiology and Intensive Care Medicine and the Professional Association of German Anesthesiologists in cooperation with the German Society for Gynecology and Obstetrics Anästh. Intensivmed. 50 (2009) S490-S495
- ↑ Cross-sectional guidelines (BÄK) on therapy with blood components and plasma derivatives. 4th revised and updated edition 2014. In: Deutsches Ärzteblatt , vol. 112, issue 6, February 6, 2015.
- ↑ Wiebke Gogarten, Hugo K. Van Aken: Perioperative thrombosis prophylaxis - platelet aggregation inhibitors - importance for anesthesia. In: AINS - Anesthesiology • Intensive Care Medicine • Emergency Medicine • Pain Therapy. Issue 04, April 2012, pp. 242-254, doi: 10.1055 / s-002-23167 .
- ↑ SA Kozek-Langenecker, D. Fries, M. Gütl, N. Hofmann, P. Innerhofer, W. Kneifl, L. Neuner, P. Perger, T. Pernerstorfer, G. Pfanner u. a .: Locoregional anesthesia with anticoagulant medication. Recommendations of the Perioperative Coagulation Working Group (AGPG) of the Austrian Society for Anaesthesiology and Intensive Care Medicine (ÖGARI). In: The anesthesiologist. Volume 54, Number 5 (2005), pp. 476-484, doi: 10.1007 / s00101-005-0827-0 .