Ticagrelor
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
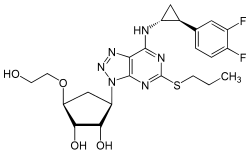
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Ticagrelor | |||||||||||||||||||||
| other names |
(1 S , 2 S , 3 R , 5 S ) -3- [7 - [(1 R , 2 S ) -2- (3,4-difluorophenyl) cyclopropylamino] -5- (propylthio) -3 H - [ 1,2,3] triazolo [4,5- d ] pyrimidin-3-yl] -5- (2-hydroxyethoxy) cyclopentane-1,2-diol |
|||||||||||||||||||||
| Molecular formula | C 23 H 28 F 2 N 6 O 4 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 522.57 g mol −1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Ticagrelor is an anti-platelet agent that prevents blood platelets from sticking together and thus prevents blood clots from forming. It is used to prevent a heart attack . The active ingredient has been approved in the European Union since 2010 and in the United States since 2011 .
Mechanism of action
Ticagrelor inhibits the ADP receptor P2Y12 on the blood platelets and thus reduces their ability to clot. A separate binding site on the receptor for the active ingredient is assumed. The binding is reversible. There is no competitive inhibition with ADP.
effect
Two hours after taking the loading dose , the peak level of the active ingredient is reached in nine out of ten people and around 70 percent inhibition of blood platelet coagulation can be determined. The bioavailability of the active ingredient is around a third, regardless of food or fluid intake. Ticagrelor has a half-life of 7 to 8 hours and is eliminated from the body by the kidneys. The platelet inhibition subsides after 1–3 days.
indication
Ticagrelor is approved for the treatment of acute coronary syndrome (unstable angina pectoris , NSTEMI, STEMI ) in combination with acetylsalicylic acid . Ticagrelor was able to reduce the rate of deaths by one percent over a year compared to a patient collective with the combination ASA / clopidogrel . Treating 54 patients with acute coronary syndrome with ticagrelor instead of clopidogrel prevents an atherothrombotic event (heart attack, stroke or unstable angina pectoris); treating 91 patients prevented cardiovascular death. Cardiovascular protection is even greater when the total daily dose of acetylsalicylic acid is a maximum of 150 mg. Even patients who have no genetic predisposition for oneThose who are clopidogrel resistant have a lower risk of cardiac death, heart attack or stroke with ticagrelor compared to clopidogrel. In the pivotal studies, ticagrelor showed an increased rate of bleeding requiring treatment, but no higher rate of severe or fatal bleeding.
Side effects and restrictions on use
A common, often self-limiting side effect is shortness of breath. Bradycardiac arrhythmias can often occur. Laboratory controls are mandatory due to the risk of kidney damage. Occasionally, gynecomastia is triggered.
For regional anesthesia procedures close to the spinal cord ( spinal anesthesia or epidural anesthesia ), ticagrelor should be discontinued five days in advance and given again at least six hours after the procedure.
Health economics
Ticagrelor, which was the first active ingredient to be recognized as having a considerable additional benefit in the AMNOG process, is significantly more expensive with annual therapy costs of 1,092 euros than the previous standard therapy with clopidogrel, which as a generic only costs 32 euros per year. A price reduction of around 20% was achieved through discount negotiations with the manufacturer. Another competing product , Prasugrel , costs only slightly less than ticagrelor. The indication and the assumption of costs were confirmed by the Federal Joint Committee in 2011. The UK's top regulatory committee, NICE , came to the same conclusion.
In 2011, the Federal Joint Committee recognized an additional benefit for the following indications: unstable angina pectoris or myocardial infarction without ST segment elevation compared to clopidogrel, myocardial infarction with ST segment elevation and percutaneous coronary intervention compared to prasugrel, if these patients are over 74 years old and are not eligible for therapy with prasugrel in combination with acetylsalicylic acid, or patients who have a history of transient ischemic attack or ischemic stroke. In these cases, Brilique is a specialty of the practice, so that in the case of a benchmark test, the corresponding regulations are deducted from the practice's budget.
Trade names
- Brilinta (US)
- Brilique (EU, CH)
- Possia (EU)
Web links
- FDA: PHARMACOLOGY / TOXICOLOGY REVIEW AND EVALUATION (PDF; 7.6 MB)
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Ticagrelor
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ Publication of the EMA Assessment Report for Brilique International non-proprietary name: ticagrelor Procedure No. EMEA / H / C / 1241. available as (pdf) ; last accessed on June 1, 2012.
- ↑ FDA press release of July 20, 2011, available as htm ; last accessed on June 1, 2012.
- ↑ JJ van Giezen, L. Nilsson, P. Berntsson, BM Wissing, F. Giordanetto: Ticagrelor binds to human P2Y (12) independently from ADP but antagonizes ADP-induced receptor signaling and platelet aggregation. In: J Thromb Haemost . 7 (9), Sep 2009, pp. 1556-1565. Epub June 23, 2009, PMID 19552634 .
- ^ S. Husted, JJ van Giezen: Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. In: Cardiovasc Ther . 27 (4), Winter 2009, pp. 259-274. PMID 19604248 .
- ↑ a b c drug telegram: Ticagrelor (Brilique) in acute coronary syndrome. at 2011; 42: 1-3, available online as html ; last accessed on June 1, 2012.
- ↑ ( page no longer available , search in web archives ) Specialist information on Brilique (PDF; 218 kB).
- ↑ L. Wallentin, S. James, RF Storey: Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. In: The Lancet . 376, 2010, pp. 1320-1328.
- ↑ Wiebke Gogarten, Hugo Van Aken: Perioperative thrombosis prophylaxis - platelet aggregation inhibitors - importance for anesthesia. In: AINS - Anesthesiology · Intensive Care Medicine · Emergency Medicine · Pain Therapy. 47, 2012, pp. 242-252, doi: 10.1055 / s-0032-1310414 .
- ↑ www.monitor-versorgungsforschung.de (accessed on March 23, 2013) .
- ^ Official announcement of the Federal Joint Committee of December 15, 2011, available online as (pdf) ; last accessed on June 2, 2012.
- ^ NICE guideline: Ticagrelor for the treatment of acute coronary syndromes . October, 2011; last accessed on June 2, 2012.
- ↑ First G-BA resolution to assess the additional benefit of a new drug: Ticagrelor has considerable additional benefit for certain patient groups. at: www.g-ba.de , accessed on May 30, 2013.
- ↑ The costs for the first newcomer have been determined. at: www.pharmazeutische-zeitung.de , accessed on May 29, 2013.