Apixaban
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
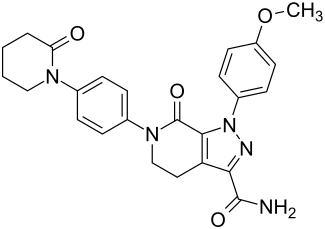
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Apixaban | |||||||||||||||||||||
| other names |
1- (4-methoxyphenyl) -7-oxo-6- [4- (2-oxopiperidin-1-yl) phenyl] -4,5,6,7-tetrahydro-1 H -pyrazolo [3,4- c ] pyridine-3-carbamide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula | C 25 H 25 N 5 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 459,50 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Apixaban is a drug from the group of anticoagulants . It inhibits thrombokinase (also factor Xa ) and thus blood clotting , which is why apixaban and the similar drugs Betrixaban , edoxaban and rivaroxaban are also referred to as direct factor Xa inhibitors ( Xabane ). The substance was developed in a cooperation between Pfizer and Bristol-Myers Squibb . Apixaban is orally effective ( e.g. as a 2.5 mg film- coated tablet ) and was approved in 2011 EU- wide under the name Eliquis for the prophylaxis of venous thromboembolism after elective (not urgently necessary) orthopedic operations; in Germany and Switzerland: after hip and knee replacement operations ( endoprostheses ) in adults. Since December 20, 2012, apixaban has also been approved in the European Union for the prevention of ischemic strokes (cerebral infarction) and systemic embolisms in adults with non-valvular atrial fibrillation . In July 2014, Apixaban was also approved in the EU for therapy and relapse prophylaxis in pulmonary embolism and deep vein thrombosis (DVT).
pharmacology
Apixaban is a direct, selective inhibitor of the enzyme factor Xa, which is involved in blood clotting . It is given orally .
Apixaban is well absorbed in the gastrointestinal tract. The bioavailability is 50%. In the liver, the substance is oxidized to a phenol derivative , with metabolism via cytochrome P450 playing a subordinate role. Apixaban reaches its maximum plasma concentration after three to four hours. The elimination Both biliary 75% and renal to 25%. Apixaban has a half-life of about 9 to 14 hours.
Apixaban is contraindicated in patients with haemophilia . The potential for interaction with other drugs is estimated to be low. Andexanet Alfa is a recombinant inhibitor of apixaban.
Risk factors for the occurrence of bleeding
In September 2013, the manufacturers of the new oral anticoagulants apixaban, dabigatran etexilate and rivaroxaban pointed out in a joint information letter agreed with the competent pharmaceutical authorities that reports of adverse drug reactions (ADRs) from clinical studies and from practice have shown that also with the new oral anticoagulants pose a significant risk of serious bleeding events, including death. In order to minimize the risk of bleeding, the prescribing physicians must individually assess the risk of bleeding of the patient and observe the information on dosage and contraindications as well as warnings and precautionary measures. Common to all new oral anticoagulants are the following contraindications:
- acute, clinically relevant bleeding
- Lesions or clinical situations considered to be a significant risk factor for major bleeding
- simultaneous use of other anticoagulants such as heparins or vitamin K antagonists (with a few exceptions).
Kidney dysfunction can also be a contraindication, but different recommendations apply to the three drugs.
rating
With non-valvular atrial fibrillation and a high risk of stroke, apixaban can be an alternative to vitamin K antagonists such as phenprocoumon in certain situations . These include difficult INR adjustment under vitamin K antagonists, high risk of hemorrhagic insults or intracerebral bleeding as well as intolerance, contraindications or interactions of vitamin K antagonists with other essential drugs. Caution is advised in patients with a high risk of bleeding, especially as long as no specific antidote was available for apixaban that can specifically neutralize the effect of apixaban in the event of severe bleeding or prior to urgent surgery.
In May 2018, the US FDA approved the recombinant protein Andexanet alfa as an antidote. In March 2019, the European approval authority EMA recommended approval (trade name in the EU: Ondexxya ) for the EU. In April 2019 it was approved by the European Commission for the European member states.
Andexanet alfa is structurally similar to factor Xa, but is enzymatically inactive. It binds apixaban and thus counteracts the binding of apixaban to the naturally occurring factor Xa.
Early benefit assessment
Since 2011, newly approved drugs with new active ingredients have had to undergo an early benefit assessment by the Federal Joint Committee (G-BA) based on Section 35a SGB V ( AMNOG ) if the pharmaceutical manufacturer wants to achieve a higher sales price than just the fixed amount . Only if there is an additional benefit can the pharmaceutical manufacturer negotiate a price with the umbrella association of statutory health insurance companies. This also applied to apixaban. In the regular course of the procedure, the Institute for Quality and Efficiency in Health Care ( IQWiG ) first submitted an assessment. It saw an added benefit of apixaban in hip replacement, whereas there was no proof of an added benefit in knee replacement surgery. The G-BA passed a resolution following this assessment ( indication of a minor additional benefit of apixaban in patients with elective hip replacement surgery ) in June 2012.
In 2012, apixaban was introduced as a new area of application, the prophylaxis of strokes and systemic embolisms in adult patients with non-valvular atrial fibrillation (NVAF) and one or more risk factors, such as a stroke or a history of transient ischemic attack, who are at least 75 years old, Hypertension, diabetes mellitus or symptomatic heart failure (NYHA class ≥II). IQWiG assessed the added benefit over vitamin K antagonists as an appropriate comparator therapy. According to the G-BA decision, there is an indication of a minor additional benefit for this indication.
Finally, in 2014, apixaban was also approved for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) as well as the prophylaxis of recurrent DVT and PE in adults. For the initial treatment and the prophylaxis to be initiated in parallel over a period of up to six months, the active ingredient was compared with the low molecular weight heparin enoxaparin and the vitamin K antagonist warfarin. For the long-term prophylaxis of recurrent DVT or PE after completion of a six-month initial treatment after which further anticoagulation is indicated, warfarin was the appropriate comparator therapy, while the G-BA saw an indication of a minor additional benefit for the Initia treatment, the additional benefit of long-term prophylaxis is not proven.
Clinical trial
The possible uses of the drug have been and are being examined in several studies. Possible indications are thrombosis prophylaxis after orthopedic operations, prophylaxis of ischemic cerebral infarcts in atrial fibrillation , prophylaxis of acute coronary syndrome and prophylaxis after thrombosis and pulmonary embolism .
The following studies are ongoing or have already been completed:
-
Completed studies:
- ADVANCE-1-3 (Apixaban Dose Orally versus Anticoagulation with Enoxaparin, Phase III studies): In the ADVANCE-1 study, thrombosis prophylaxis with apixaban 2 × 2.5 mg / day with enoxaparin 2 × 30 mg / day ( US dosage regimen) in patients after implantation of a knee joint prosthesis . Therapy started about 12 hours after the operation. The aim of the study was to demonstrate that apixaban was not inferior . This did not work. One result of the study was that significantly fewer bleeding complications occurred during therapy with apixaban . The ADVANCE-2 study also examined the effectiveness of thrombosis prophylaxis after the implantation of a knee joint prosthesis. The start of therapy with apixaban was 12–24 hours after the end of the operation, with enoxaparin 12 hours before the operation. Apixaban (2 × 2.5 mg / day) was shown to be superior to therapy with enoxaparin (1 × 40 mg / day). A similar study result was shown in the ADVANCE-3 study. Here, the thrombosis prophylaxis in patients with hip replacement was compared. The start of therapy and dose were applied as in the ADVANCE-2 study. Bleeding complications did not occur more frequently during therapy with apixaban.
- APPRAISE-2: In this study, therapy with apixaban (2 × 5 mg po / day) was compared to placebo therapy in addition to monotherapy with acetylsalicylic acid or to dual platelet inhibition with acetylsalicylic acid and clopidogrel in patients with acute coronary syndrome. Due to a significant increase in the risk of bleeding under therapy with apixaban, the study was terminated early.
- AVERROES (Apixaban Versus ASA to Reduce the Rate of Embolic Stroke, Phase III study): In this study, therapy with apixaban (2 × 2.5-5 mg po / day) with acetylsalicylic acid (1 × 81-324 mg po / day) in patients with atrial fibrillation in whom therapy with vitamin K antagonists was not carried out for various reasons. A significant superiority of apixaban in the prevention of ischemic strokes and other systemic embolisms with a simultaneous non-significant increase in bleeding complications was demonstrated, so that the study was terminated prematurely.
- ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation, Phase III study). In this double-blind randomized study, patients with atrial fibrillation were shown to be non-inferior to therapy with apixaban (2 × 5 mg po / day) compared to warfarin (target INR: 2.0-3.0). The endpoint of the study was the occurrence of an ischemic or hemorrhagic stroke or a systemic embolism. After a median follow-up of 1.8 years, this endpoint was observed with a frequency of 1.27% / year in the apixaban group and 1.60% / year in the warfarin group (reduction of the relative risk by −21% ). This proved that apixaban was not inferior to warfarin in a statistically significant manner. Essentially, the rate of hemorrhagic stroke was reduced, but not the rate of ischemic stroke. The absolute frequency of all bleeding complications was reduced by 7.7%, the clinically relevant severe bleeding was 31% less common with apixaban (2.13% / year vs. 3.09% / year).
- AMPLIFY : In this phase III study, therapy with apixaban (2 × 10 mg / day for 7 days, followed by 2 × 5 mg / day for 6 months) was started in 5395 patients with acute deep vein thrombosis and / or acute pulmonary embolism Compared to standard therapy with enoxaparin followed by warfarin (bridging therapy). The result of the study is that apixaban is not inferior to conventional therapy. There was significantly less bleeding.
Contraindications
For regional anesthesia procedures close to the spinal cord ( spinal anesthesia or epidural anesthesia ), apixaban should be discontinued 26–30 hours beforehand and given again at least four to six hours after the procedure. Apixaban should be discontinued for at least 24 hours prior to planned surgery in procedures with a low risk of bleeding. In interventions in which a high risk of bleeding cannot be excluded or is unacceptable, the time is at least 48 hours, which corresponds to at least four half-lives in healthy kidneys. With renal insufficiency , this time can be significantly longer.
Web links
- Entries in the NIH study registry
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Apixaban
literature
- Weitz, Thromb Haemost 2006; 96: 274-84
- Jennifer Carreiro & Jack Ansell: Apixaban, an oral direct Factor Xa inhibitor: awaiting the verdict. Expert Opin. Investig. Drugs (2008) 17 (12): 1937-1945
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Lisa Nainggolan: Apixaban better than European enoxaparin regimen for preventing VTE. Retrieved October 3, 2011 .
- ↑ New drugs (PDF; 150 kB). Information from the Drugs Commission of the German Medical Association (AkdÄ), as of March 5, 2013, last accessed September 17, 2013.
- ↑ European Commission Approves Eliquis (apixaban) for the Treatment of Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE), and prevention of recurrent DVT and PE . Press release from the manufacturing company Bristol-Myers Squibb. Retrieved September 16, 2014.
- ^ A b D. Garcia, E. Libby, MA Crowther: The new oral anticoagulants. In: Blood Volume 115, number 1, January 2010, pp. 15-20, doi : 10.1182 / blood-2009-09-241851 , PMID 19880491 . (Review).
- ↑ Jörg Braun: Blood, blood products and coagulation disorders. In: Jörg Braun, Roland Preuss (Ed.): Clinic Guide Intensive Care Medicine. 9th edition. Elsevier, Munich 2016, ISBN 978-3-437-23763-8 , pp. 539-579, here: p. 562 ( Apixaban ).
- ↑ Information letter on risk factors for the occurrence of bleeding from September 5th, 2013. (PDF; 2.5 MB) Retrieved on September 9th, 2013 .
- ↑ New Medicines (PDF; 150 kB) Information from the Medicines Commission of the German Medical Association (AkdÄ) , as of March 5, 2013.
- ↑ The first antidote for apixaban and rivaroxaban comes to Europe. Deutsche Apotheker Zeitung online, March 4, 2019, accessed on August 9, 2019 .
- ↑ Overview of Ondexxya and why it is approved in the EU , EMA April 2019, accessed on August 9, 2019
- ↑ Ondexxya - andexanet alfa , EMA, accessed on August 9, 2019
- ↑ European Commission Grants Conditional Marketing Authorization for Portola Pharmaceuticals' Ondexxya ™ (andexanet alfa), the First and Only Antidote for the Reversal of Factor Xa Inhibitors , PM Portola, April 26, 2019, accessed April 29, 2019
- ↑ Accelerated approval for Antidot , Pharmazeutische Zeitung of March 1, 2019, accessed on August 9, 2019
- ↑ thepharmaletter.com Retrieved May 17, 2018.
- ↑ aerzteblatt.de Retrieved on May 17, 2018.
- ↑ A11-30 Apixaban - benefit assessment according to § 35a Social Code Book V (dossier assessment); Accessed March 23, 2020.
- ↑ Benefit assessment procedure for the active ingredient apixaban (prophylaxis of venous thromboembolism); Accessed March 23, 2020.
- ↑ A12-20 Apixaban - Benefit assessment according to Section 35a SGB V (dossier assessment); Accessed March 23, 2020.
- ↑ Benefit assessment procedure for the active ingredient apixaban (new field of application: prophylaxis of strokes and systemic embolisms); Accessed March 23, 2020.
- ↑ A14-28 Apixaban (approval extension) - Benefit assessment according to Section 35a SGB V (dossier assessment); Accessed March 23, 2020.
- ↑ Benefit assessment procedure for the active ingredient apixaban (new area of application: treatment and prophylaxis of venous thrombosis and pulmonary embolism); Accessed March 23, 2020.
- ↑ MR Lassen, GE Raskob, A. Gallus, G. Pineo, D. Chen, RJ Portman: apixaban or enoxaparin for thromboprophylaxis after knee replacement. In: The New England journal of medicine Volume 361, Number 6, August 2009, pp. 594-604, doi : 10.1056 / NEJMoa0810773 . PMID 19657123 .
- ↑ MR Lassen, GE Raskob, A. Gallus, G. Pineo, D. Chen, P. Hornick: apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomized double-blind trial. In: The Lancet Volume 375, number 9717, March 2010, pp. 807-815, doi : 10.1016 / S0140-6736 (09) 62125-5 , PMID 20206776 .
- ^ MR Lassen, A. Gallus, GE Raskob, G. Pineo, D. Chen, LM Ramirez: Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. In: The New England journal of medicine Volume 363, Number 26, December 2010, pp. 2487-2498, doi : 10.1056 / NEJMoa1006885 , PMID 21175312 .
- ^ A b J. Ansell: Warfarin versus new agents: interpreting the data. In: Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program Volume 2010, 2010, pp. 221-228, doi : 10.1182 / asheducation-2010.1.221 , PMID 21239798 .
- ↑ APPRAISE Steering Committee and Investigators, Alexander JH, Becker RC, Bhatt DL, et al .: Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation 2009; 119: 2877-2885, doi : 10.1161 / CIRCULATIONAHA.108.832139 , PMID 19470889 .
- ↑ SJ Connolly, J. Eikelboom et al. a .: Apixaban in patients with atrial fibrillation. In: The New England journal of medicine Volume 364, Number 9, March 2011, pp. 806-817, doi : 10.1056 / NEJMoa1007432 , PMID 21309657 .
- ↑ Granger CB, Alexander JH, McMurray JJV, et al. for the ARISTOTLE Committees and Investigators: Apixaban versus Warfarin in Patients with Atrial Fibrillation. N Engl J Med 2011,365 (11): 981-992, PMID 21870978 .
- ↑ G. Agnelli, HR Buller et al. a .: Oral apixaban for the treatment of acute venous thromboembolism. In: The New England Journal of Medicine . Volume 369, Number 9, August 2013, pp. 799-808, doi : 10.1056 / NEJMoa1302507 , PMID 23808982 .
- ↑ Wiebke Gogarten, Hugo Van Aken: Perioperative thrombosis prophylaxis - platelet aggregation inhibitors - importance for anesthesia In: AINS - anesthesiology · intensive care medicine · emergency medicine · pain therapy. 47, 2012, pp. 242-252, doi : 10.1055 / s-0032-1310414 .
- ↑ Specialist information Eliquis https://www.eliquis.de/EliquisDE_landingPage?ReturnUrl=EliquisDE_Fachinformationen_new ( page no longer available , search in web archives ).