Gyrase inhibitors
Gyrase inhibitors are chemical compounds that inhibit the activity of gyrase - enzymes slow or prevent. In prokaryotes, gyrases are used to supercoil the DNA, which both saves space and improves partial readability of the DNA. By inhibiting gyrase, the DNA loses its compact structure and expands, which ultimately leads to the cell bursting. The inhibition of bacterial gyrase is the principle behind some antibiotics .
The chemical starting point for the development of gyrase inhibitors were nalidixic acid ( diazanaphthalenes ) and the similar pipemidic acid ( pyridopyrimidines ). Common to all representatives of the gyrase inhibitors is a heterocyclic structure with a nitrogen atom in the ring (position 1), a carboxy group in position 3 and a keto group in position 4. Gyrase inhibitors have both bacteriostatic and bactericidal effects, depending on the dose . In the bicyclic system, diazanaphthalenes have at least two (nalidixic acid) and triazanaphthalenes (or pyridopyrimidines ) three (pipemidic acid) nitrogen atoms.
Classification
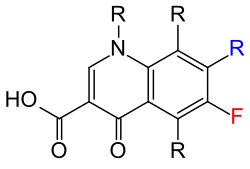
From a chemical point of view, there are different groups:
- Quinolone antibiotics (a subgroup of fluoroquinolones )
- Cinnoline (see cinnolin )
- Naphthyridines (see nalidixic acid )
- Pyridopyrimidines (see pipemidic acid )
- Aminocoumarins
Among the gyrase inhibitors, the fluoroquinolones have the greatest therapeutic importance, which is why both names are often used synonymously. According to the classification by the Paul Ehrlich Society, fluoroquinolones are divided into four subgroups depending on their spectrum of activity .
Since newer fluoroquinolones not only inhibit gyrase (= bacterial topoisomerase II ), but also other types such as topoisomerase IV, the term "gyrase inhibitor" is no longer used in the international specialist literature.
Individual evidence
- ↑ Ernst Mutschler , Gerd Geisslinger, Heyo K. Kroemer , Sabine Menzel, Peter Ruth: Mutschler drug effects. Pharmacology - Clinical Pharmacology - Toxicology. 10th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2012, ISBN 3-80-472898-7 . P. 770.