Diazanaphthalenes
In chemistry, the diazanaphthalenes form a group of organic compounds that belong to the heterocycles (more precisely: heteroaromatics ). They consist of a naphthalene ring in which two carbon atoms have been replaced by nitrogen. Their different arrangement results in ten isomers with the empirical formula C 8 H 6 N 2 .
The entire group is divided into two subgroups:
- four benzodiazines with both N atoms in only one ring: quinazoline , quinoxaline , cinnoline and phthalazine
- six naphthyridines or pyridopyridines with one nitrogen atom per ring
Isomers
| Surname | Structural formula | Subgroup |
|---|---|---|
| 1,2-diazanaphthalene ( cinnoline ) |
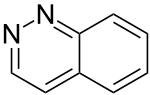 |
Benzodiazine |
| 1,3-diazanaphthalene ( quinazoline ) |
 |
Benzodiazine |
| 1,4-diazanaphthalene ( quinoxaline ) |
 |
Benzodiazine |
| 1,5-diazanaphthalene | 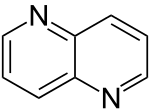 |
Naphthyridine |
| 1,6-diazanaphthalene |  |
Naphthyridine |
| 1,7-diazanaphthalene |  |
Naphthyridine |
| 1,8-diazanaphthalene |  |
Naphthyridine |
| 2,3-diazanaphthalene ( phthalazine ) |
 |
Benzodiazine |
| 2,6-diazanaphthalene | 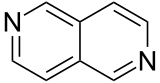 |
Naphthyridine |
| 2,7-diazanaphthalene | 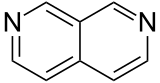 |
Naphthyridine |
Individual evidence
- ↑ Entry on naphthyridines. In: Römpp Online . Georg Thieme Verlag, accessed on August 28, 2014.
- ↑ Desmond J. Brown, Jonathan A. Ellman, Edward C. Taylor: The Chemistry of Heterocyclic Compounds, The Naphthyridines , John Wiley & Sons, 2007 ( limited preview in Google Book Search).
- ^ William W. Paudler, Thomas J. Kress: Naphthyridine chemistry. IX. Bromination and animation of the 1, X-naphthyridines , J. Org. Chem. , 1968 , 33 (4), pp. 1384-1387 ( doi : 10.1021 / jo01268a018 ).