Grepafloxacin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Grepafloxacin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 22 FN 3 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 359.39 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
206-208 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Grepafloxacin is a broad spectrum antibiotic belonging to the group of fluoroquinolones . Antibiotics from the group of fluoroquinolones are a subgroup of the quinolones , they act as gyrase inhibitors against gram-positive bacteria ( hemophilus , legionella , mycoplasma , chlamydia, etc.), especially in respiratory infections.
Grepafloxacin was patented in 1989 by the pharmaceutical company Warner-Lambert (now Pfizer ). After some patients as a side effect of the long QT syndrome , an arrhythmia occurred with seven deaths associated with taking the drug, it was taken by the manufacturer end of October 1999 worldwide from the market.
Stereochemistry
Grepafloxacin contains a stereocenter and consists of two enantiomers. This is a racemate , i.e. a 1: 1 mixture of ( R ) - and ( S ) -form:
| Grepafloxacin enantiomers | |
|---|---|
 CAS number: 146761-68-4 |
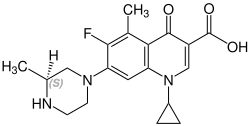 CAS number: 146761-69-5 |
literature
- W. Forth, D. Henschler, W. Rummel: General and special pharmacology and toxicology . 9th edition. URBAN & FISCHER, Munich 2005, ISBN 3-437-42521-8 .
Individual evidence
- ↑ a b Entry on grepafloxacin. In: Römpp Online . Georg Thieme Verlag, accessed on May 30, 2014.
- ↑ There is not yet a harmonized classification for this substance . A labeling of grepafloxacin in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on May 26, 2020, is reproduced from a self-classification by the distributor .
- ↑ FURTHER CRASH CANDIDATES? CARDIOTOXICITY OF GYRASIS inhibitors . Remedy telegram . S. 120 November 1999. Retrieved June 4, 2009.