Allostery
Allostery ( Greek . Ἄλλως allos "different" and στερεός Stereos "rigid") is a term used in biochemistry , of the protein function is concerned.
It is used differently in literature. It initially applies to proteins that bind certain molecules ( effectors ) with a specific regulatory effect at a different location than their substrate ( enzyme ) or their central ligand ( carrier or receptor ). Cases in which the binding strength depends on the number of substrate or ligand molecules already bound are also included.
Allostery initially meant the change in conformation while influencing the active center / binding center . Some authors take the view that a cooperative interaction between separate subunits of an ( oligomeric ) protein is definitely necessary for the allosteric effect . According to this view, no allosteric effects should occur with monomeric proteins. However, changes in the spatial structure of such proteins are known due to small molecules that can have an influence on the active center. It has therefore become common practice to subsume these phenomena under the term allostery. For the example of phosphofructokinase , this means that here every polypeptide chain is to be regarded as a fusion of two subunits (C and R). Each of these subunits binds ATP , C as a substrate (coenzyme) and R as an allosteric inhibitor.
This principle is also used in supramolecular chemistry to change the strength of the bond to substrate molecules by inserting an effector and thus to establish a molecular switch.
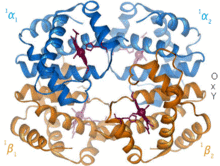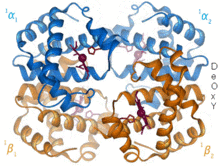
The prototype of an allosteric protein is hemoglobin , in which the strength of the oxygen (O 2 ) binding depends on a number of effectors, but in particular on how many of the four O 2 binding sites are already occupied. At higher oxygen concentrations / loads, the transition from a low-affinity "T-state" (T for tense) to the high-affinity "R-state" (R for relaxed) occurs. = relaxed). The fact that subsequent O 2 molecules are increasingly bound is also referred to as positive cooperativity . The Hill coefficient is a measure of cooperativity.
See also
literature
- Jeremy M. Berg, John L. Tymoczko, Lubert Stryer : Biochemistry. 6 edition, Spektrum Akademischer Verlag, Heidelberg 2007. ISBN 978-3-8274-1800-5 .
- Donald Voet, Judith G. Voet: Biochemistry. 3rd edition, John Wiley & Sons, New York 2004. ISBN 0-471-19350-X .
- Bruce Alberts , Alexander Johnson, Peter Walter, Julian Lewis, Martin Raff, Keith Roberts: Molecular Biology of the Cell , 5th Edition, Taylor & Francis 2007, ISBN 978-0815341062 .
Individual evidence
- ↑ C. Kremer, A. Lützen: Artificial Allosteric Receptors . In: Chem Eur J.. . 19, No. 20, May 2013, pp. 6162-6196. doi : 10.1002 / chem.201203814 .