2-phenylethanol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
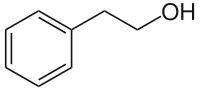
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 2-phenylethanol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 8 H 10 O | |||||||||||||||||||||
| Brief description |
colorless liquid smelling of rose oil |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 122.17 g mol −1 | |||||||||||||||||||||
| Physical state |
liquid |
|||||||||||||||||||||
| density |
1.02 g cm −3 |
|||||||||||||||||||||
| Melting point |
−27 ° C |
|||||||||||||||||||||
| boiling point |
220 ° C |
|||||||||||||||||||||
| Vapor pressure |
8 Pa (20 ° C) |
|||||||||||||||||||||
| solubility |
|
|||||||||||||||||||||
| Refractive index |
1.5317 (20 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||||||||
2-Phenylethyl alcohol is a chemical compound from the group of alcohols that smells like roses.
Occurrence
Phenylethyl alcohol is a component of a large number of natural essential oils, such as those from hyacinths , carnations , roses and geraniums .
Extraction and presentation
Phenylethyl alcohol can be obtained through
- Friedel-Crafts acylation of ethylene oxide with benzene
- Hydrogenation of styrene oxide on Raney nickel
- Reduction of phenylacetic esters such as phenylacetic acid ethyl ester with sodium in absolute ethanol
- Conversion of phenylmagnesium bromide and ethylene oxide with subsequent hydrolysis
properties
2-Phenylethanol is a colorless liquid with a scent of rose petals and honey and a sharp, burning taste. The substance is sensitive to light and also decomposes when exposed to air.
use
Phenylethyl alcohol is an important ingredient in fragrances that smell of roses and is widely used to simulate sweet floral scents such as orange blossom, jasmine, geranium and many more. It is stable to alkalis and therefore ideally suited as a fragrance in soaps .
It also serves as a starting material for organic (especially fragrance) syntheses. Its esters with lower fatty acids and the alkyl ethers (so-called KEWDA ethers) are also valuable fragrances and aromas. Phenylacetaldehyde can be produced from phenylethyl alcohol by mild oxidation and phenylacetic acid can be produced by further oxidation .
Since phenylethanol also has a bacteriostatic effect, it can also be used as a preservative , disinfectant and antiseptic .
safety instructions
2-Phenylethanol is also very irritating to the eyes in low concentrations. If larger amounts are absorbed via the respiratory tract or skin, disorders of the central nervous system and the gastrointestinal tract can occur.
Individual evidence
- ↑ Entry on PHENETHYL ALCOHOL in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ Entry on 2-phenylethanol. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ a b c d e f g h i j k Entry on 2-phenylethanol in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-34.
- ^ A b David B. Troy, Paul Beringer: Remington The Science and Practice of Pharmacy . Lippincott Williams & Wilkins, 2006, ISBN 978-0-7817-4673-1 , pp. 1066 ( limited preview in Google Book search).
- ↑ Data sheet 2-phenylethanol, ≥99.0% (GC) from Sigma-Aldrich , accessed on January 13, 2017 ( PDF ).
- ↑ H. Hager, Fv Bruchhausen, P. Surmann, E. Nuremberg: Hagers Handbook of Pharmaceutical Practice . Springer, 1999, ISBN 3-540-52641-2 , p. 171.
