Pyruvate dehydrogenase E1
| Pyruvate dehydrogenase E1 | ||
|---|---|---|

|
||
| Ribbon model of the tetramer according to PDB 1NI4 | ||
| Mass / length primary structure | 1380 = 2 * 361 + 2 * 329 amino acids | |
| Secondary to quaternary structure | 2α + 2β | |
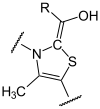
| Cofactor | Thiamine diphosphate | |
| Identifier | ||
| Gene name (s) | PDHA1 , PDHA2 , PDHB | |
| Enzyme classification | ||
| EC, category | 1.2.4.1 , oxidoreductase | |
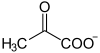
| Substrate | Pyruvate + lipoyllysine-PDHE2 | |
| Products | S-acetyldihydrolipoyllysine -PDHE2 + CO 2 | |
| Occurrence | ||
| Parent taxon | Creature | |
Pyruvate dehydrogenase E1 ( PDHE1 ) is the name for the subunit E1 of the pyruvate dehydrogenase enzyme complex . PDHE1 catalyzes the transfer of an acetyl residue to the lipoyllysine bound to subunit E2 , releasing one molecule of carbon dioxide . E1 itself consists of two α and two β subunits. There is a second isoform of α in humans that is specifically expressed in the testes.
Mutations in the genes that code for α and β (80 are known in α alone) can cause PDHE1 deficiency up to and including Leigh's syndrome and lactic acidosis .
Catalyzed reaction
The decarboxylation of pyruvate takes place at the thiamine as the catalytic center, which forms an atomic bond with pyruvate, so that hydroxy - ethylidene - thiamine - pyrophosphate is formed with the elimination of CO 2 .
This hydroxy-ethylidene residue (syn. Acetaldehyde ) of the TPP is taken over by the α-lipoic acid ( oxidation ). It is covalently bound to the lipoat trans acetylase subunit. The result is S-acetyl hydrolip (oat / onamid).
It appears that the two thiamine molecules in the tetramer do not go through the aforementioned reaction sequence at the same time. A related movement of the tetramer was found in a crystal study.
According to a study on hamsters, the testicular-specific isoform of the enzyme plays a role in capacitation .
regulation
PDHE1 is inactivated by phosphorylation of the α unit or activated by dephosphorylation. The corresponding enzyme is PDH kinase ( EC 2.7.11.2 ), which is itself part of PDH (= autophosphorylation). Increased activity can already be triggered by increased muscle work, whereby a dependence of PDH phosphatase on the mitochondrial Ca 2+ concentration is being discussed. An inhibition by sepsis could be shown in rats.
Individual evidence
- ↑ a b UniProt P08559
- ↑ Cameron JM, Levandovskiy V, Mackay N, Tein I, Robinson BH: Deficiency of pyruvate dehydrogenase caused by novel and known mutations in the E1alpha subunit . In: Am. J. Med. Genet. A . 131, No. 1, November 2004, pp. 59-66. doi : 10.1002 / ajmg.a.30287 . PMID 15384102 .
- ↑ Han Z, Gorbatyuk M, Thomas J, Lewin AS, Srivastava A, Stacpoole PW: Down-regulation of expression of rat pyruvate dehydrogenase E1alpha gene by self-complementary adeno-associated virus-mediated small interfering RNA delivery . In: Mitochondrion . 7, No. 4, July 2007, pp. 253-9. doi : 10.1016 / j.mito.2007.02.003 . PMID 17392036 . PMC 1973157 (free full text).
- ↑ Ciszak EM, Korotchkina LG, Dominiak PM, Sidhu S, Patel MS: Structural basis for flip-flop action of thiamin pyrophosphate-dependent enzymes revealed by human pyruvate dehydrogenase . In: J. Biol. Chem. . 278, No. 23, June 2003, pp. 21240-6. doi : 10.1074 / jbc.M300339200 . PMID 12651851 .
- ↑ Kumar V, Rangaraj N, Shivaji S: Activity of pyruvate dehydrogenase A (PDHA) in hamster spermatozoa correlates positively with hyperactivation and is associated with sperm capacitation . In: Biol. Reprod. . 75, No. 5, November 2006, pp. 767-77. doi : 10.1095 / biolreprod.106.053587 . PMID 16855207 .
- ↑ Rassow J et al. 2008. Biochemistry. 2nd edition, Stuttgart: Thieme Verlag, 109.
- ↑ Stellingwerff T, Watt MJ, Heigenhauser GJ, Spriet LL: Effects of reduced free fatty acid availability on skeletal muscle PDH activation during aerobic exercise. Pyruvate dehydrogenase . In: Am. J. Physiol. Endocrinol. Metab. . 284, No. 3, March 2003, pp. E589-96. doi : 10.1152 / ajpendo.00418.2002 . PMID 12556353 .
- ↑ Vary TC: Sepsis-induced alterations in pyruvate dehydrogenase complex activity in rat skeletal muscle: effects on plasma lactate . In: Shock . 6, No. 2, August 1996, pp. 89-94. PMID 8856841 .