Chitin
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Chitin | ||||||
| other names |
|
||||||
| CAS number | 1398-61-4 | ||||||
| Monomer | Acetylglucosamine | ||||||
| Molecular formula of the repeating unit | C 8 H 13 NO 5 | ||||||
| Molar mass of the repeating unit | 203.19 g mol −1 | ||||||
| PubChem | 6857375 | ||||||
| Type of polymer | |||||||
| Brief description |
Polysaccharide , whitish solid |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Chitin ( Greek χιτών chitón , German 'shell, shell' ) is next to cellulose the most common polysaccharide and is used for structure formation. It differs from cellulose in that it has an acetamide group . It is present in mushrooms and in arthropods (Articulata) and molluscs before. In fungi, it forms one of the main components of the cell wall . In annelid worms it occurs in the mouth. In arthropods , it is the main component of the exoskeleton . It has also been found in vertebrates, such as bony fish and slime fish (Blenniidae) such as the gray slime fish ( Paralipophrys trigloides ).
Chitin is the starting material for the technical production of chitosan and glucosamine .
Occurrence
In the animal kingdom, chitin, in combination with protein and calcium carbonate , is widespread as a component in the exoskeleton of many arthropods , especially the classes (and superclasses) of insects , arachnids , millipedes and crustaceans . The molluscs it is as part of the radula and in Schulp some cephalopods to be found.
In the mushroom kingdom, chitin is found in a number of lower fungi as well as in stand mushrooms , ashlar mushrooms and mucorales as a cell wall component with proteins and glucans , although it does not occur in all of these fungi. Even in close relatives, the occurrence of chitin in the cell wall can differ considerably.
Structure and properties
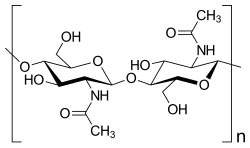
Chitin is a polysaccharide made up of acetylglucosamine units (more precisely: 2-acetamido-2-deoxy-D-glucopyranose, or N-acetyl-D-glucosamine for short, abbreviation: GlcNAc). The acetylglucosamine units are linked by β-1,4-glycosidic bonds - this is the same type of bond as that of the glucose molecules in cellulose. Chitin can therefore be understood as a variant of cellulose in which the hydroxyl groups in position 2 of the monomer units have been replaced by acetamido groups. This enables a stronger hydrogen bond between adjacent polymers, which makes chitin harder and more stable than cellulose. However, natural chitin is usually not a uniform polymer, but a mixture of random copolymers of D-glucosamine (GlcN) and N-acetyl-D-glucosamine (GlcNAc), that is, not every amino group is acetylated.
The degree of acetylation determines its properties in addition to the degree of polymerization (chain length) and the chain folding. The transition to chitosan , which has significantly fewer (ideally no) acetyl groups, is therefore fluid. If the degree of acetylation is higher than 50%, it is usually referred to as chitin ; if it is lower , it is usually referred to as chitosan .
Chitin occurs naturally in at least two conformations : the arthropod chitin occurs mainly in the form of α- chitin, in molluscs in the form of β- chitin. A mixture of α- and β-chitin, which occurs in beetle larvae and cephalopods, is sometimes referred to as γ -chitin.
Chitin is colorless. The well-known brown color (as well as the firmness) of insect shells is caused by sclerotin , a structural protein.
Chitin is largely insoluble in aqueous, weakly ionic and health-tolerable organic solvents, in strongly ionic solvents the "solubility" is based on depolymerization. “Soluble chitin” is mostly a chitin hydrochloride , some of which are even soluble in water.
biosynthesis
In nature, chitin forms complex structures that are formed in a multi-stage process. Chitin molecules are synthesized by transglycosylations , membrane-bound enzymes known as chitin synthetase EC 2.4.1.16 , which use uridine diphosphate -N-acetylglucosamine (UDPGlcNAc) as substrate. In mushrooms, for example, this happens in special vesicles called chitosomes. The chitin molecules or chito oligomers are secreted into the extracellular space . Various modifications take place outside the cells that affect the properties. One of them is the partial hydrolysis by chitinases EC 3.2.1.14 . In addition to hydrolase, chitinases also have transglycosidase activity, so that chitin can be linked to glucans . Another modification is the partial deacetylation by special deacetylases. Some unmodified chitin molecules crystallize and are partially covalently linked with other chitin molecules via proteins. The resulting supramolecular structure matures through further cross-linking and the incorporation of various substances.
Biological importance
Chitin has been produced by living things for at least 500 million years. Contrary to popular belief, chitin is not responsible for making (insect) armor hard. Chitin is responsible for its softness and flexibility. It is only in interaction with the structural protein sclerotin that the cuticle of the insect becomes hard and stable. In crustaceans , lime is also stored to increase the hardness.
Chitin is the second most common biopolymer after cellulose. An estimate for the annual biosynthesis is 10 10 to 10 11 tons. The main part is made up of the small crustaceans of the zooplankton (e.g. krill ).
Chitin from aquatic arthropods and fungi is u. a. degraded by Vibrio cholerae , the cholera pathogen, with the help of the enzyme chitinase .
Active ingredients that inhibit chitin synthesis are known as chitin inhibitors and are used to combat insects and fungi.
Story of discovery
Chitin was scientifically described for the first time in 1811 by Henri Braconnot (director of the Botanical Garden of Nancy ) as a substance (from mushrooms), but not yet under this name, but as "fungin". The French Antoine Odier gave the name in 1823: he took the Greek word for “tunic” or “sheath”, based on the wing covers of the cockchafer , in which he had found the substance. The clarification of the chemical structure of chitin took place in 1929 by Albert Hofmann (known as the discoverer of LSD) as part of his doctoral thesis.
Medical importance
Chitin is also a component of important pathogens; it is found in the cell walls of pathogenic fungi, in the vagina and pharynx of filariae, as well as in the eggs of parasitic worms. Mammals and plants have chitin-degrading chitinases for defense. In patients with Gaucher's disease , extremely high enzyme levels are found, which are used to control therapy. In other lysosomal storage diseases and patients with sarcoidosis increased enzyme levels found in the blood. In severe asthma , increased chitinase levels can be detected in serum and lung tissue .
use
Although chitin as a biopolymer has very good mechanical properties and, alongside cellulose and lignin, is one of the most common natural polymers, the range of uses is comparatively small. Chitosan , which is derived from chitin, is commercially produced from the shell remains of shrimp and used primarily as a "fat blocker" in the food sector and as a filter material for water extraction or in sewage treatment plants and as a raw material for fibers, foams, membranes and foils ( bio-based plastic ). Chitosan is also used in toothpastes (Chitodent), as a paper and cotton additive, and for removing cloudiness in the beverage industry. Chitosan is being researched in the pharmaceutical industry in order to use it for microencapsulation and the targeted release of pharmacological agents, including as a vector for gene therapy.
Chitin is also the starting material for the technical production of glucosamine , a natural component of cartilage and synovial fluid . Technically produced glucosamine is used in the pharmaceutical industry and the like. a. in remedies against osteoarthritis .
Web links
- Unexpected allergy potential - study: Biopolymer chitin causes allergy-like inflammation. Wissenschaft.de (Summary of an article from Nature : Tiffany A. Reese, Hong-Erh Liang, Andrew M. Tager, Andrew D. Luster, Nico Van Rooijen, David Voehringer, Richard M. Locksley: Chitin induces accumulation in tissue of innate immune cells associated with allergy. In: Nature , advance online publication, April 22, 2007)
- Information about chitin. Heppe Medical Chitosan
Individual evidence
- ↑ Entry on CHITIN in the CosIng database of the EU Commission, accessed on February 25, 2020.
- ↑ a b c Entry on chitin at TCI Europe, accessed on June 18, 2019.
- ↑ a b entry on chitin. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2012.
- ^ GP Wagner, J. Lo, R. Laine, M. Almeder: Chitin in the epidermal cuticle of a vertebrate (Paralipophrys trigloides, Blenniidae, Teleostei). In: Experientia . 49, 1993, pp. 317-319, doi: 10.1007 / BF01923410 .
- ↑ Albert Gossauer: Structure and reactivity of biomolecules . Verlag Helvetica Chimica Acta, Zurich 2006, p. 347, ISBN 978-3-906390-29-1 .
- ↑ How do chitin chains connect? ( Memento from November 20, 2008 in the Internet Archive )
- ↑ Ram Minke, John Blackwell: The structure of α-chitin . In: Journal of Molecular Biology . tape 120 , no. 2 , 1978, p. 167-181 , doi : 10.1016 / 0022-2836 (78) 90063-3 .
- Jump up ↑ Yukie Saito, Takeshi Okano, Françoise Gaill, Henri Chanzy, Jean-Luc Putaux: Structural data on the intra-crystalline swelling of β-chitin . In: International Journal of Biological Macromolecules . tape 28 , no. 1 , 2000, pp. 81-88 , doi : 10.1016 / S0141-8130 (00) 00147-1 .
- ↑ NE Dweltz: The structure of β-chitin . In: Biochimica et Biophysica Acta . tape 51 , no. 2 , 1961, p. 283-294 , doi : 10.1016 / 0006-3002 (61) 90169-X .
- ^ Samuel M. Hudson, David W. Jenkins: Encyclopedia of Polymer Science and Technology . John Wiley & Sons, Inc., Hoboken, NJ 2002, ISBN 978-0-471-44026-0 , chapter Chitin and Chitosan , doi : 10.1002 / 0471440264.pst052 .
- ↑ Molecule of the month: chitin / chitosan. In: chemieonline.de. Retrieved February 9, 2017 .
- ↑ J. Ruiz-Herrera, AD Martínez-Espinoza: Chitin biosynthesis and structural organization in vivo. In: EXS Volume 87, 1999, pp. 39-53, PMID 10906950 .
- ↑ Chitin, over 500 million years old, discovered in an ancient sponge. In: tu-freiberg.de. TU Bergakademie Freiberg , December 17, 2013, accessed on February 9, 2017 .
- ↑ H. Ehrlich, J. Keith Rigby, JP Botting, MV Tsurkan, C. Werner, P. Schwille, Z. Petrášek, A. Pisera, P. Simon, VN Sivkov, DV Vyalikh, SL Molodtsov, D. Kurek, M Kammer, S. Hunoldt, R. Born, D. Stawski, A. Steinhof, VV Bazhenov, T. Geisler: Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta . In: Scientific Reports . tape 3 , 2013, doi : 10.1038 / srep03497 , PMID 24336573 .
- ↑ FL Campbell: The Detection and Estimation of Insect Chitin; and the Irrelation of "Chitinization" to Hardness and pigmentation of the cuticle of the American Cockroach, Periplaneta Americana L . In: Annals of the Entomological Society of America . tape 22 , no. 3 , 1929, pp. 401-426 , doi : 10.1093 / aesa / 22.3.401 .
- ↑ W. ARBIA et al .: chitin Recovery Using Biological Methods . In: Food Technol. Biotechnol. , 51 (1), 2013, pp. 12-25.
- ↑ Hiroshi Tamura, Tetsuya Furuike: Encyclopedia of Polymeric Nanomaterials . Ed .: Shiro Kobayashi, Klaus Müllen. Springer, Berlin, Heidelberg 2014, ISBN 978-3-642-36199-9 , chapter Chitin and Chitosan , p. 1 , doi : 10.1007 / 978-3-642-36199-9_322-1 .
- ↑ A. Hofmann: About the enzymatic breakdown of chitin and chitosan. University of Zurich, 1929, OCLC 601730630 .
- ↑ Guo Yufeng et al .: Elevated plasma chitotriosidase activity in various lysosomal storage disorders . In: Journal of Inherited Metabolic Disease , 1995, PMID 8750610 .
- ↑ Grosso, Bargagli et al .: Serum levels of chitotriosidase as a marker of disease activity and clinical stage in sarcoidosis . In: Scandinavian Journal of Clinical and Laboratory Investigation , 2004, 64 (1), pp. 57-62, PMID 15025429 .
- ↑ Geoffrey L. Chupp et al .: A Chitinase-like Protein in the Lung and Circulation of Patients with Severe Asthma . In: N Engl J Med . No. 357 , 2007, p. 2016–2027 , doi : 10.1056 / NEJMoa073600 .



