kidney
The kidney ( Latin ren , usually only in the plural renes , derived adjective renalis ; ancient Greek νεφρός nephrós ) is a paired organ of the urinary system for the preparation of urine and regulation of the water and electrolyte balance of vertebrates . In the two kidneys, parts of the blood below a certain size are filtered off, most of the molecules important for the organism are reabsorbed, others are also secreted and the aqueous solution is concentrated before it is excreted. Nephrology as a branch of internal medicine and urology are primarily concerned with diseases of the kidneys .
Functions of the kidneys are:
- Excretion of end products of metabolism , the so-called uremic substances and toxic substances from the body through formation of urine , which is eventually excreted by the urinary system of the body;
- Osmoregulation (balancing the water balance);
- Volume regulation (long-term blood pressure adjustment);
- Regulation and control of the composition of the urine and controlling the electrolyte balance and the acid-base balance .
The kidney is also an important organ for the body's intermediate metabolism (it carries out gluconeogenesis ). The kidneys also produce hormones , such as erythropoietin for blood formation , and are the place where peptide hormones are broken down. Conversely, many kidney functions are controlled by hormones; The renin formed in the kidney itself can cause the blood pressure to be high enough for its blood flow.
The basic functional unit of the kidney is the nephron , which consists of kidney corpuscles and tubules . The basic functionality of a nephron can be roughly divided into two processes:
In the first process, which takes place in the kidney corpuscle, the primary urine is pressed out of the blood by cross-flow filtration . During this filtration, components above a certain size, including blood cells and larger molecules, are retained. This means that the ultrafiltrate only contains the low molecular weight components of the blood plasma, including those that are to be excreted. However, this primary urine also - and predominantly - contains numerous substances that are valuable for the body. Galenos already thought about kidney filtration. William Bowman proved that glomeruli and tubules form a functional unit.
In a second process, which then takes place in the kidney tubules, valuable substances such as sugar, amino acids and electrolytes are brought back into the bloodstream in a controlled manner and reabsorbed ( reabsorption ). Furthermore, a large part of the filtered water is absorbed, which should not be lost to the body. These resorption processes take place in different sections of the adjoining, tube-like tubular system - and in addition, such active secretion of substances that are subject to excretion into the urine. The kidney tubules concentrate the primary urine into the secondary urine ( terminal urine ), which collects in the renal pelvis, the beginning of the urinary tract .
From here, the urine is continuously directed via the ureter (ureter) to the urinary bladder . It is occasionally excreted from the bladder through the urethra .
In an adult, around 1800 liters of blood flow through the kidneys ( renal blood flow ) every day , which is roughly 300 times the blood volume of the body. From this, the two organs filter around 180 liters of primary urine daily ( glomerular filtration ), which is concentrated in less than two liters of terminal urine (urine).
Macroscopic anatomy
In humans
Location and neighborhood relationships
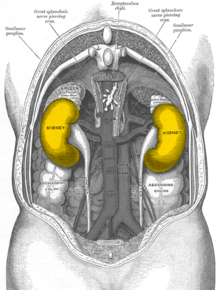
In humans, the kidneys are located retroperitoneally (behind the peritoneum), on both sides of the spine , which they do not protrude forward, below the diaphragm , in the lumbar fossa. The kidneys are located approximately at the level of the twelfth thoracic vertebra to the third lumbar vertebra , the right one (because of the large right lobe of the liver) about half a vertebra lower than the left. The upper poles of the kidneys (see under shape) are about 7 cm apart, the lower ones about 11 cm. The longitudinal axes of both organs consequently point upwards towards the center of the body. The lower poles of the kidneys are 3 cm on the right, 4 cm on the left, 2.5 cm and 3 cm on the woman, respectively, from the iliac crest, but they can also reach the iliac crest variably. The position of the kidneys depends on the breath. When you inhale, they move caudally, as does the diaphragm. In the newborn, the kidney is always comparatively larger than other structures and therefore usually towers above the iliac crest.
Apart from the adrenal glands, the kidneys have contact surfaces with several organs in the abdomen, separated by the fat capsule. The contact surfaces are different for the left and right kidney: The left kidney of the stomach , spleen , the Milzgefäßen ( A. and splenic ), pancreatic tail ( tail of the pancreas ), and colon ( transverse colon ) superimposed. With a triangular surface that is in contact with the peritoneum , it forms part of the back surface of the omental bursa . The right kidney is mainly covered by the liver , but also by the colon and duodenum ( pars transversum duodeni ). Because of the space required by the right lobe of the liver on the right in the body (with the impressio renalis ), the right kidney is lower than the left. The crescent-shaped adrenal gland sits like a cap on both kidneys.
The nerve nerve subcostal , nerve iliohypogastricus and nerve ilioinguinalis run the back of the kidney, crossing in close proximity and can be also affected in diseases. This can lead to sensations that are assigned to the innervation areas of these nerves, including pain in the lower abdominal area.
Shape, color and size
The kidneys are bean-shaped and brownish red. They have a length of 10 to 12 cm, a width of 5 to 6.5 cm and a thickness of 3 to 5 cm (note: 12 cm × 6 cm × 3 cm). The mass of a kidney varies between 120 and 200 g. Usually the left kidney is slightly larger and heavier. If one kidney is significantly reduced in size or is absent, the other is usually enlarged. In humans, two so-called kidney poles point upwards and downwards, two surfaces to the front and back (ventral and dorsal) and two edges to the medial and lateral sides. The outwardly directed edge is convex, the medially directed edge is concave and forms an indentation in which the renal hilum , the entry and exit port of the ducts, lies.
Renal hilum and ducts
At the renal hilus, the vena renalis , arteria renalis and the ureter as well as some lymphatic vessels and nerves branch from anterior to dorsal . The hilus expands inside the kidney into the renal sinus , which is filled by the renal pelvis (urinary tract) and fatty tissue.
Each kidney is usually of a (very rarely more) directly from the aorta springing renal artery with blood supplies. The renal artery branches off from the aorta on both sides at the level of the superior mesenteric artery, points downwards and divides in front of the hilum into an anterior and posterior main trunk (ramus anterior et posterior), which are named after their position in relation to the renal pelvis and the segmental arteries submit:
Four segmental arteries arise from the anterior main trunk in front of the hilus, the A. segmenti superioris, A. segmenti anterioris superioris, A. segmenti anterioris inferioris, A. segmenti inferioris. The posterior main trunk gives off a posterior segmental artery and supplies only one segment on the back of the kidney. The arteriae segmentorum are followed by the arteriae interlobaren, then the arteriae arcuatae, then the arteriae interlobulares (also Arteriae corticales radiatae), which finally give off the vasa afferentia for the kidney corpuscles of the nephrons. For a more detailed description of the arterial supply, see the section Feinbau and the article Nephron .
The renal artery and each of its end branches are end arteries, there are no anastomoses, so that the closure of a branch leads to the death of the kidney tissue it supplies (necrosis, kidney infarction).
The renal vein carries the blood directly into the inferior vena cava . In the body, the aorta is on the left and the inferior vena cava on the right, which is why the left renal vein is longer than the right. It lies in front of the aorta, below the exit of the superior mesenteric artery (→ Nutcracker syndrome ) and accommodates the testicular vein or left ovary.
The urine released by the kidney into the renal pelvis is transported through the ureter (ureter) to the urinary bladder .
Lymph capillary networks inside the kidney collect the kidney's lymph and form a few hilar lymph vessels on the hilus.
The sympathetic nerves of the kidney originate as postganglionic fibers from the celiac plexus and run with the renal artery. In addition to the kidney parenchyma, they supply the pain-sensitive capsule. The parasympathetic nerves of the kidney originate as rami renales directly from the vagus nerve (Xth cranial nerve).
Cases
The sheaths of the kidneys include the capsula fibrosa, capsula adiposa and fascia renalis (= Gerota fascia):
Both kidneys are each encased by a thin, firm and smooth connective tissue organ capsule ( capsula fibrosa ). It contains very few elastic fibers and is hardly stretchable.
Along with the adrenal glands follows a loose Fettgewebskörper of structural fat , the fat capsule , which embeds the kidney and abpolstert. The capsula adiposa is more strongly developed on the back and sides than on the abdomen and continues into the fat of the renal sinus inside the kidney. The fat body can be broken down if there is severe malnutrition.
All of this envelops the renal fascia, a fascia sack that surrounds the kidney, adrenal gland and the fat body to the front, side and back, but is not closed at the top and bottom medially. Behind the renal fascia sac lies the retrorenal fat ( massa adiposa pararenalis ), in which nerves of the lumbar plexus run.
Internal structure: bark and pith
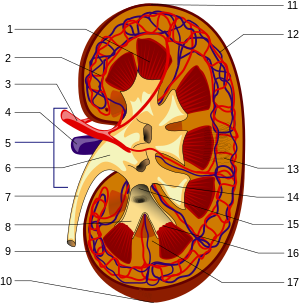
1. Renal medulla with medullary cones ( Pyramides renales )
2. Vas afferens
3. Renal artery ( Arteria renalis )
4. Renal vein ( Vena renalis )
5. Hilum renale
6. Renal pelvis ( Pelvis renalis )
7. Ureter ( ureter )
8. Small renal calyxes ( Calices minores renales )
9. Renal capsule ( Capsula fibrosa renalis )
10. Lower renal pole ( Extremitas inferior )
11. Upper renal pole ( Extremitas superior )
12. Vas efferens
13. Nephron
14. Renal indentation ( Sinus renalis )
15. Large kidney calyxes ( Calices majores renales )
16. Tips of the medullary cones ( Papillae renales )
17. Bertin column ( Columna renalis )
The kidney parenchyma, the actual organ mass of the kidney, is divided into the outer renal cortex ( cortex renalis ) and the renal medulla ( medulla renalis ), which is directed inwards towards the hilum . The pith has the shape of pyramids (10 to 12 pith pyramids or kidney pyramids ) with their base pointing outwards and their tip pointing inwards towards the hilum. These tips, the papillae , reach freely into the cavity of the renal calyx ( calix renalis ), which combine in variable form to form the renal pelvis ( pelvis renalis ), from which the ureter emerges. In this arrangement, urine flows from the papillae towards the ureter.
The renal cortex lies like a cap between the bases of the medullary pyramids and the organ capsule (subcapsular part), but reaches between the pyramids in columnar sections ( Columnae renales , also called Columnae renales Bertini or Bertinian columns after the French anatomist Exupère Joseph Bertin since 1744 ) Renal sinus . The subcapsular part of the cortex is traversed by clearly visible, fine lines, the medullary rays ( Radii medullares ), which radiate radially from the medullary pyramids in the direction of the organ capsule and are part of the pith. In the medulla itself, due to their slightly different color, an outer medulla, consisting of an outer and an inner stripe, and an inner medulla facing the renal pelvis can be distinguished.
The structural division of the renal medulla into inner and outer zones as well as the division into inner and outer strips of the outer zone was described for the first time by the anatomist Karl Peter (1870–1955), based on examinations initiated by his Würzburg teacher Philipp Stöhr in 1904/05.
In other mammals
The basic position is also typical in the other mammals , here the kidneys (according to the horizontal body orientation) lie behind the diaphragm. In many mammals, the right kidney is a little further forward. In ruminants , the development of the rumen causes the left kidney to be shifted to the right, behind the right kidney ( physiological migrating kidney ).
The kidneys are structured differently in the individual mammals. In its simplest form, the kidney consists of individual, conical kidney lobes ( Lobi renales ). This multi-lobed kidney is typical of marine mammals and bears . Each kidney lobe consists of a cortical cap and a medullary pyramid that ends in a renal papilla ( renal papilla , the pointed end of the cone).
In most mammals, these kidney lobes (6 lobes in humans) fuse to varying degrees. The merging cortical caps form the renal cortex ( Cortex renis ), the pyramids the renal medulla ( Medulla renis ).
In cattle, only the central parts of the individual kidney lobes fuse, creating furrows on the surface and also preserving the kidney papillae. This type of construction is called a multi-waxy, furrowed kidney . In the meantime, this form also occurs in the fetal development of the kidney in mammals, which are characterized by further fusion processes. The human newborn also has a polygonal, furrowed kidney.
In primates (including humans) and pigs, the bark parts fuse completely after birth, so that the organ surface appears smooth. However, the individual papillae are retained. One speaks of a polygonal smooth kidney .
In most mammals, the individual kidney papillae now also merge to form a renal crest ( crista renalis ), so that one speaks of a one- black, smooth kidney .
Feinbau
The fine structure of the kidney is characterized by a highly differentiated tubular system and a specifically adapted blood supply. Due to the development of the embryo , the tubular system can be divided into two parts, the nephron and the collecting tube . Both form a functional unit. The last nephron section, that is to say close to the collecting tube, is embryologically assigned to the collecting tube.
Blood supply to the kidney
The kidneys are normally perfused by about 20% of the cardiac output (in adults about 1000 ml / min). Approximately 20% of the renal plasma flow is filtered into Bowman's space. Renal perfusion leads to glomerular filtration ( GFR ). Therefore, the GFR is largely proportional to the cardiac output CO . That is why the stage of renal insufficiency is never less than the stage of heart failure .
Branches
The segmental arteries (see above) continue to divide. An interlobaric artery supplies two adjacent medullary pyramids and corresponding cortical areas. It runs in the bark columns along the sides of the pyramids towards the bark, but branches out at the base of the pyramid into arteriae arcuatae . These run in an arch at the medullary- cortex border and give off the arteriae corticales radiatae running radially upwards through the cortex at a right angle and the arteriae rectae also almost at right angles in the medullary direction .
First and second capillary bed
From these the Vasa afferentia emerge, each of which is divided into a capillary ball, the glomerulus (see below). From this first capillary area the still oxygen-rich blood converges again in the vas efferens . From there the blood enters a second capillary bed, this time to supply the kidney tissue. A distinction must be made between two cases, depending on the position of the glomerulus: From superficial glomeruli, which are located in the upper area of the cortex towards the organ capsule , the blood enters the peritubular capillary network of the cortex, which spins the tubules located there. However, the vessels supplying the marrow arise from juxtamedullary glomeruli, which lie deeper towards the medullary-cortical border.
The supply of the marrow
These capillary vessels for supplying the medulla are the vasa recta , which often descend straight to the tip of the papilla and rise again in the opposite direction. There are numerous cross-connections between descending and ascending legs. The special vascular architecture of the marrow is of great functional importance for the ability of the kidney to concentrate urine. With the help of the counter-current principle , the kidney creates a considerable osmotic gradient towards the tip of the papilla (see below), which would be washed out if the medulla were supplied with a normal capillary network. The price for this, however, is a very poor oxygen supply to the renal medulla, since the oxygen from the oxygen-rich, descending limb of the vasa recta can diffuse directly above into the ascending, oxygen-poor thigh.
Venous system
Both capillary networks finally reach the venous system of the kidney, which - with the exception of the glomerula and their afferent and efferent arterioles - is structured analogously to the arterial system.
Nephron
The kidney consists of numerous units, the nephrons, in which urine is produced. Each of the human kidneys contains 1 to 1.4 million nephrons. The nephron itself consists of a renal corpuscle ( corpusculum renis ) and a tubular apparatus ( tubules ).
In the kidney corpuscle is the glomerulus (also called the glomerulum ), a tangle of vessels through whose fenestrated capillary walls the primary urine is filtered off. The primary urine passes at the urinary pole (see illustration) from the kidney corpuscle into the proximal tubule and into Henle's loop, where it is concentrated according to the countercurrent principle. This is followed by the distal tubule and a collecting tube ( tubulus renalis colligens ).
Glomerulus in the scanning electron microscope (SEM)
image width approx. 115 µmGlomerulus with broken capillary in the SEM
image width approx. 11.5 µm
development
During the embryonic development, the amniotes (umbilical animals) develop three generations of kidneys: anterior kidney (pronephros), urnal kidney (mesonephros) and posterior kidney (metanephros). The anterior kidney does not yet take on any function in the embryo. This task is only started by the urn kidney and taken over by the post-kidney. The tissue of the post-kidney eventually grows into the final kidney.
The post-kidney arises from two systems: the metanephrogenic blastema , the later urinary section, and the ureter bud , the later urinary drainage and urine volume controlling section. The renal parenchyma with the nephrons, into which the branches from the aorta sprout, arise from the former. Persistence of the fetal blastemic tissue can lead to nephroblastomatosis .
The ureter, the renal pelvis with the kidney calyxes, the collecting ducts and the last sections of the nephron adjoining the collecting duct arise from the ureteral bud.
The kidneys experience an ascent due to the growth in length of the embryo (ascensus). In doing so, they move upwards from the area of the pelvis. If the two lower poles of the kidney grow together, a single horseshoe kidney can develop. If a kidney does not rise, it remains in the area of the pelvis ( pelvic kidney ). If the kidney rises too high, it can lie in the chest (intrathoracic kidney).
Initially, several mesonephric arteries supply the urinary kidney, most of which regress and usually only one renal artery remains. However, a second renal artery is relatively common. Accessory renal arteries are used when there is an additional artery that opens into the hilar, and an aberrant artery when the vessel does not open at the hilar but independently - often at a pole. More than two renal arteries can occur, but are very rare
function
Functions of the kidney
The kidney is involved in the following body functions:
- Regulation of the body's water balance.
- Long-term regulation of blood pressure .
- Excretion of urinary (e.g. uric acid , urea , creatinine ) and toxic (e.g. drugs) substances.
- Regulation of the body's acid-base balance . The pH value of the blood must only fluctuate within a narrow range, since larger changes in the direction of acidic or alkaline values lead to death.
- Regulation of the content of dissolved electrolytes in the blood ( homeostasis ): sodium , potassium , calcium , magnesium , phosphate , bicarbonate .
- Formation of various hormones: renin (enzyme, short-term blood pressure regulation), erythropoietin (stimulation of blood formation ), calcitriol (vitamin D, involved in calcium metabolism ), kinins and prostaglandins .
- Significant participation in the synthesis of glucose (grape sugar) called gluconeogenesis , in addition to the liver .
Measurement of kidney performance
The function of the kidney can be estimated from the amount of urine, the urine concentration and the concentration of urinary substances (creatinine, urea, uric acid, potassium) in the blood.
The exact performance of the kidneys is determined by renal clearance . There are different procedures for this:
- The renal clearance is a measure of the elimination of a substance in the blood plasma and thus for clarifying function of the kidney. If the clearance drops, i.e. if the kidney's performance decreases, it is called renal insufficiency .
- The inulin clearance measures the filtration capacity of the kidneys. For this purpose, the patient is administered inulin and measured how much of the administered substance is excreted per time. Since inulin is filtered but not reabsorbed, the inulin clearance is identical to the glomerular filtration rate (GFR). For healthy adolescents, the value is around 125 ml / min. A decrease in the value indicates a disorder in kidney function (renal insufficiency). With increasing age, the GFR decreases physiologically to 60–65 ml / min. This is important when dosing drugs that are excreted via the kidneys, as elderly patients often have to reduce the dose because of the lower GFR .
- The creatinine clearance is due to their ease of implementation in the clinic preferred to the inulin clearance. The excretion of creatinine is measured, which roughly corresponds to that of inulin. The creatinine plasma level, the value of which depends on the muscle mass, fluctuates only slightly, which is what makes this measurement possible in the first place. It is also advantageous that the infusion , which is required when measuring the inulin clearance, is omitted.
Autoregulation of renal blood flow
The driving force of the filtration process is the blood pressure in the glomerulus vessels of the first capillary bed . The body's (systemic) blood pressure is normally subject to typical fluctuations over the course of a day, is lower during sleep , higher during physical exertion or stress and can be permanently high in certain diseases ( arterial hypertension ). Adequate pressure is necessary for filtration in the glomeruli, ideally only slightly fluctuating. The kidneys themselves have the ability to regulate the blood pressure in the glomerular capillary network even without nervous impulses and to keep the glomerular filtration rate so largely constant that even strong fluctuations in systemic blood pressure hardly have any effect. This autoregulation of the kidney is called the Bayliss effect .
The autoregulation is mediated locally by means of pressure sensors and takes place through adapted changes in the vessel tension or vessel width in the blood vessels leading to and from the kidney corpuscle. When the systemic blood pressure rises , the renal arteries are narrowed so that the renal blood flow hardly increases and the pressure in the afferent vessels of the kidney corpuscles behind it does not become excessive. If the filtration pressure is too low, the resistance in the (efferent) vessel going from the glomerulus is increased and at the same time decreased in the supplying one. This means that the effective filtration pressure can also be regulated independently of the renal blood flow. The mean glomerular capillary pressure is approximately 50 mmHg.
Normal blood pressure fluctuations have little effect on kidney blood flow. In this way, fluctuations in systolic blood pressure between 80 and 180 mmHg have no effect on glomerular filtration performance. To a certain extent, the kidneys constantly monitor the systemic blood pressure with their sensitive pressure sensors and can intervene in a regulating manner in the event of an excessive drop (cf. blood pressure regulation of the kidneys).
Tubuloglomerular Feedback (TGF)
As tubuloglomerular feedback (TGF) refers to a mechanism with which the filtration of a single nephron is regulated in the kidney. The TGF postulates an inverse behavior of glomerular filtration and tubular reabsorption and thus to a certain extent a proportionality between primary urine formation and urine production.
When the NaCl content in the distal tubule (middle section) increases, a sensor function of the macula densa , part of the juxtaglomerular apparatus , leads to a reduction in the glomerular filtration rate of the same nephron. This is achieved through a vasoconstriction (vasoconstriction) of the arterioles leading to the kidney corpuscles ( vasa afferentia ) mediated by the mesangium .
Strictly speaking, this is a physiological regulation mechanism that is supposed to protect the single nephron from hyperfiltration and is "incorrectly" activated in the event of acute kidney failure by the fact that the NaCl absorption is severely impaired by the tubular damage. This leads to an increased flow rate in the distal tubule and / or to an increased supply of NaCl in the area of the macula densa, which ultimately leads to the triggering of the tubuloglomerular feedback.
Methods of examination of the kidney
- Physical examination
- Touch
- Knocking
- Laboratory tests
-
Urinalysis
- Test sticks for nitrite, leukocytes , protein, blood, sugar etc.
- Urine sediment
- Creatinine Clearance
- Electrolytes
- The urine is also assessed visually and by smell
- Blood test
- Stone examinations
-
Urinalysis
- Imaging
- Ultrasonic
- X-ray contrast agent display of the kidney = iv pyelogram
- CT of the kidney
- Magnetic resonance imaging of the kidney
- Angiography of the kidney
- Nuclear medicine procedures
- histological examinations
Diseases of the kidney
In acute kidney disease or chronic kidney failure, abnormal changes in the kidney tissue can affect the glomerula ( glomerulonephritis ) or the renal tubules ( tubulointerstitial kidney disease ). In the former, more autoimmune processes play a role, in the latter intoxication and infections (acute bacterial infections in particular). In addition, both can be affected by autoimmune or metabolic systemic diseases. Genetically determined diseases mostly affect the function of the tubules. The various processes hardly differ clinically, a distinction is made between acute and chronic kidney failure or acute and chronic glomerulonephritis. If left untreated, they lead to glomerulosclerosis and renal insufficiency with the need for dialysis . There are also investment errors, kidney tumors, kidney stones.
Serious damage to the kidneys, on the other hand, results in disorders of the blood pressure and hormone regulation of the organism. There is renal hypertension , renal vitamin D deficiency and secondary hyperparathyroidism , in severe chronic renal failure for uremic syndrome with organ damage and, among other itching. The damage can possibly be slowed down by a diet low in salt and protein and a lot of drinking, or dialysis therapy becomes necessary.
Systematics
- Kidney malformations
- Numerical anomalies: e.g. B. missing or additional kidney, s. Renal agenesis , renal hypoplasia
- Position, fusion and rotation anomalies: kidney misalignment, migrating kidney, crossed kidney dystopia, horseshoe kidney, malrotation of the kidneys.
- Malformations of the renal vessels
- Malformations of the calyx system: z. B. calyx diverticulum or megacalicosis .
- Hereditary or genetic cystic kidney disease:
- Autosomal recessive polycystic kidney disease (ARPKD) → cyst kidney
- Autosomal dominant polycystic kidney disease (ADPKD) → cyst kidney
- Juvenile nephronophthisis
- medullary cystic disease
- Congenital Nephrosis Syndrome
- Malformation syndromes such as von Hippel-Lindau syndrome , tuberous brain sclerosis
- Non-hereditary cystic kidney disease:
- simple kidney cyst
- Parapelvic kidney cysts
- Benign multilocular kidney cyst
- Multicystic kidney dysplasia
- Medullary sponge kidney
-
Glomerulonephritis / glomerulopathy (autoimmune inflammation of the kidneys)
- acute (with nephritic syndrome )
- chronic (with nephrotic syndrome )
- C1q nephropathy
- IgA nephritis
- Focal-segmental glomerulonephritis , see under Nephrotic Syndrome
- membranous glomerulonephritis
- Membranoproliferative glomerulonephritis types I , II and III , possibly due to Alport syndrome (defect of type IV collagen , associated with hematuria, progressive kidney failure and inner ear hearing loss )
- Minimal-Change Glomerulonephritis
-
Renal tubulo-interstitial disease
- acute
- bacterial pyelonephritis (inflammation of the kidney pelvis)
- viral ( hantaviruses )
- parainfectious ( streptococci , Epstein-Barr virus )
- allergic / toxic
- chronic
- Analgesic nephropathy and other intoxications / hypersensitivity reactions
- Sarcoid
- acute
- Systemic diseases with kidney involvement
- Vasculitis (autoimmune blood vessel inflammation)
- Other vascular changes
- Metabolic impairments
- Hereditary kidney disease
- tubular dysfunction
- glomerular diseases
- Kidney stones and nephrocalcinosis
- Kidney depression (colloquially " wandering kidney ")
-
tumor
- Malignant: renal cell carcinoma
- benign: z. B. Angiomyolipoma , Oncocytoma
- mechanical compression ( Page kidney )
Syndromes
- Acute kidney failure
- Chronic kidney failure
- Hepatorenal syndrome
- Nephrotic Syndrome and Nephritic Syndrome
- Renal hypertension
- Renal anemia
- nephrogenic vitamin D deficiency (secondary hyperparathyroidism )
- Renal osteodystrophy
- Uremia
- Polyuria , nocturia , polydipsia , oliguria , anuria
Effects of losing a kidney
After losing a kidney, for example after a nephrectomy (for example after an accident, because of a hypernephroma or for a kidney transplant ), the remaining kidney can achieve up to 80% of the filtration capacity of both kidneys. This hyperfiltration is achieved by hypertrophy of the glomeruli . This does not adversely affect the remaining kidney for decades.
Kidney as a food
Pork, veal and lamb kidneys are mainly used as food. They are mostly prepared in the form of ragouts .
literature
- Johanna Bleker : The History of Kidney Diseases. Mannheim 1972 (= medical history series of studies Boehringer Mannheim . Volume 2).
- Joachim Frey : Diseases of the kidneys, the water and salt balance, the urinary tract and the male genital organs. In: Ludwig Heilmeyer (ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition, ibid. 1961, pp. 893-996.
- Frank H. Netter , Eckehard Renner: color atlases of medicine. Volume 2: Kidney and Urinary Tract. Thieme, Stuttgart 1983, ISBN 3-13-524102-5 .
- Uwe Gille: urinary and sexual system, urogenital apparatus. In: FV. Salomon, Hans Geyer, Uwe Gille (Ed.): Anatomy for veterinary medicine. 2nd, expanded edition. Enke-Verlag, Stuttgart 2008, ISBN 978-3-8304-1075-1 .
- A. Werner Mondorf, Jürgen E. Scherberich: The normal kidney. Picture atlas. Vieweg Verlag, Wiesbaden / Braunschweig 1986, ISBN 3-528-07926-6 .
Web links
- Unifr.ch: Detailed description of the urinary system ( Memento from November 16, 2010 in the Internet Archive )
- Electron microscopic images
Individual evidence
- ^ Johanna Bleker : The history of kidney diseases , Boehringer Mannheim , Mannheim 1972, p. 15.
- ↑ Named after the Romanian anatomist Dimitrie Gerota
- ↑ Reinhard Hildebrand: Bertin, Exupère Joseph. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 170.
- ↑ Wolfram F. Neiss: On the genesis of the "Investigations into the structure and development of the kidney" (1909): A handwritten letter from Karl Peters to Philipp Stöhr senior. In: Würzburger medical history reports , Volume 6, 1988, pp. 293-300; here: p. 293 and 297 f.
- ↑ Harrison's Internal Medicine. 19th edition. McGraw-Hill, Berlin 2016, ISBN 978-3-88624-560-4 , electronic chapter 332e.
- ↑ Hyewon Hahn et al .: Quiz Page January 2009: Retro Cardiac Mass Identified at Birth . In: American Journal of Kidney Diseases . No. 53 , 2009, p. A27-A28 ( article ).
- ↑ Sahib J. Tuteja, Bence Forgacs: Multiple renal arteries Newe England Journal of Medicine 2019, Volume 381, Issue 9 of August 29, 2019, page 862, DOI: 10.1056 / NEJMicm1902894
- ^ A b Ulrich Welsch, Wolfgang Kummer, Thomas Deller: Textbook Histology. 4th edition. Elsevier, Urban & Fischer, Munich et al. 2015, ISBN 978-3-437-44433-3 , p. 457.