Dextrans
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
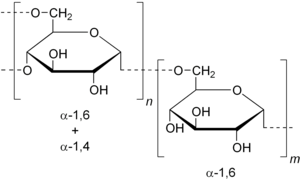
| Structure section: shown are α-1,4 and α-1,6 linkages to neighboring molecules | |||||||
| General | |||||||
| Surname | Dextran | ||||||
| CAS number | 9004-54-0 | ||||||
| Monomer | glucose | ||||||
| Molecular formula of the repeating unit | C 6 H 10 O 5 | ||||||
| Molar mass of the repeating unit | 162.14 g mol −1 | ||||||
| Type of polymer | |||||||
| Brief description |
white to off-white odorless solid |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| solubility |
soluble in water |
||||||
| safety instructions | |||||||
|
|||||||
| Toxicological data | |||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Dextrans are high-molecular, branched, neutral biopolysaccharides that yeast and bacteria serve as reserve substances . Since the polymers only consist of glucose units, they belong to the homoglycans . Natural dextrans have molecular weights between 10,000 and 50,000,000 Da . They are produced by bacteria of the genus Leuconostoc ( L. mesenteroides and L. dextranicum ) using enzymes outside the cells ( extracellular ) from sucrose .
Properties and structure
Dextrans are water-soluble, the solubility depending on the molecular mass. This creates highly viscous, slimy liquids. The colloid osmotic pressure of a six percent aqueous solution of dextrans with a molar mass of about 75,000 Da corresponds to that of the blood, which is why it can be used as a blood plasma substitute . Low molecular weight dextrans act as platelet aggregation inhibitors .
Dextrans are highly branched polysaccharides. The glycosidic linkage to the neighboring glucose molecules can take place via a 1.6-, 1.4- or 1.3-, rarely also a 1,2-linkage.
Molar mass distribution
For pharmaceutical applications of dextrans a certain molar mass distribution is specified in the European Pharmacopoeia and a test method for it. The test is carried out by means of exclusion chromatography .
use
Dextrans are used:
- in 6 percent solution as a blood plasma substitute. The viscosity and colloid osmotic pressure of this solution correspond to those of the blood serum .
- as a carrier in affinity chromatography (with dextran-epichlorohydrin copolymer )
- for the purification of mononuclear cells of the peripheral blood (with sucrose-epichlorohydrin copolymer )
- in freeze-drying as a stabilizing additive
- in modified form in gel permeation chromatography (with dextran-epichlorohydrin copolymer)
- as a measurement standard in membrane technology
They are also used in products such as:
- Paints
- Means for soil improvement
- Detergents
- Film
- cosmetics
- Aids for paper and textile production
- Adhesives and glues
Use in microsurgery
Low molecular weight dextrans are used in surgery to minimize the risk of thrombosis in the blood vessels . The antithrombotic effect of dextrans is based on the increase in osmolarity and the consequent increase in plasma volume , which reduces viscosity and increases blood flow. Dextrans also reduce the activation of factor VIII protein ( Von Willebrand factor protein ), which is necessary for blood clotting . They also have an inhibiting effect on the α2-antiplasmin, whereby they activate plasminogen .
The length of time it remains in the body depends on the molar mass. At 40,000 Da the dextrans remain in the bloodstream for two to four hours, at 70,000 Da for four to six hours. Higher dextrans are only very poorly discharged via the kidneys, which is why they stay in the body considerably longer. During this time the thrombolytic properties remain.
Chromatography
Cross- linked dextran-epichlorohydrin copolymers were developed by Jerker Porath in 1957 . They are used for gel permeation chromatography (GPC), as they are arranged in a defined three-dimensional network due to the crosslinking (e.g. with epichlorohydrin ) and thus form pores . Molecules that are larger than the pores migrate when the solvent passes through such a column , since they are not incorporated into the pores. Smaller molecules can interact with them and accordingly move more slowly. It is therefore possible to separate molecules according to size and shape. This is well suited for large biomolecules that can be separated without great effort. Cross-linked dextran is also used for immunoprecipitation and as a stationary phase in affinity chromatography .
Various dextran derivatives are used in ion exchange chromatography , e.g. B. Diethyl aminoethyl dextran (DEAE dextran, an anion exchanger), carboxymethyl dextran (CM-dextran) or dextran sulfate.
brand names
Cross-linked dextrans are also known under the brand name Sephadex from Pharmacia . Sephadex is a acronym of se paration Pha rmacia dex tran. Sephadex types modified by hydoxypropylation are also produced for lipophilic and hydrophilic (as Sephadex LH 20 and Sephadex LH 60) gel chromatography. These products also swell in various organic solvents such as methanol and chloroform , thus expanding the range of application.
literature
- Entry to dextrans. In: Römpp Online . Georg Thieme Verlag, accessed on December 18, 2014.
Individual evidence
- ↑ a b c d e Dextran 500 data sheet (PDF) from Carl Roth , accessed on July 30, 2017.
- ↑ Entry on dextrans in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Science-Online-Lexika: Entry on Dextrans in the Lexikon der Chemie. Retrieved January 24, 2009.
- ↑ a b c d Alexander Aloy: Surgical Intensive Care Medicine: Compendium for Practice. Springer, 2007, ISBN 978-3-211-29679-0 , p. 295.
- ^ A b J. R. Siewert, M. Allgöwer: Chirurgie. 7th edition. Springer, 2000, ISBN 3-540-67409-8 , p. 439.
- ↑ European Pharmacopoeia , Deutscher Apotheker Verlag Stuttgart, 6th edition, 2008, pp. 78–80, ISBN 978-3-7692-3962-1 .
- ↑ P. Roderick, G. Ferris, K. Wilson, H. Halls, D. Jackson, R. Collins, C. Baigent: Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anesthesia as thromboprophylaxis. In: Health Technol Assess. Volume 9 (49), 2005, pp. Iii-iv, ix-x, pp. 1-78. PMID 16336844 . PDF ( Memento of the original from May 11, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Jerker Porath , Per Flodin: Gel filtration: a method for desalting and group separation. In: Nature. Volume 183 (4676), 1959, pp. 1657-1659. PMID 13666849 .
- ↑ J. Porath: Gel filtration of proteins, peptides and amino acids. In: Biochim Biophys Acta. Volume 39, 1960, pp. 193-207. PMID 14434211 .
- ^ P. Andrews: Estimation of the molecular weights of proteins by Sephadex gel filtration. In: Biochem J. Vol. 91 (2), 1964, pp. 222-233. PMID 4158310 ; PMC 1202876 (free full text).
- ^ J. Porath: From gel filtration to adsorptive size exclusion. In: J Protein Chem. (1997), Vol. 16 (5), pp. 463-468. PMID 9246630 .
- ↑ J. Porath, EB Lindner: Separation methods based on molecular sieving and ion exclusion. In: Nature. Volume 191, 1961, pp. 69-70. PMID 13737223 .
- ^ A. Tiselius, J. Porath, PA Albertsson: Separation and fractionation of macromolecules and particles. In: Science. Volume 141 (3575), 1963, pp. 13-20. PMID 13985156 .
- ^ R. Axén, J. Porath: Chemical coupling of enzymes to cross-linked dextran ('Sephadex'). In: Nature (1966), Vol. 210 (5034), pp. 367-369. PMID 5963228 .
- ^ R. Axén, J. Porath, S. Ernback: Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. In: Nature. Volume 214 (5095), 1967, pp. 1302-1304. PMID 6056841 .
- ↑ J. Porath, R. Axén: Immobilization of enzymes to agar, agarose, and Sephadex supports. In: Methods Enzymol. Volume 44, 1976, pp. 19-45. PMID 1021680 .
- ↑ Sephadex LH 20 properties