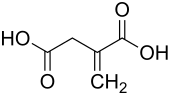
Itaconic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Itaconic acid | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 6 O 4 | |||||||||||||||
| Brief description |
white, flammable, hygroscopic, odorless powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 130.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.63 g cm −3 |
|||||||||||||||
| Melting point |
162-167 ° C |
|||||||||||||||
| boiling point |
Decomposition from 268 ° C |
|||||||||||||||
| pK s value |
3.84 and 5.55 |
|||||||||||||||
| solubility |
moderate in water (83 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Itaconic acid (C 5 H 6 O 4 ) is an organic dicarboxylic acid . It is created as one of three products in the distillation of citric acid . Itaconic acid is soluble in water, ethanol and acetone .
properties
Itaconic acid occurs at normal temperatures in the solid state as a white and almost odorless powder. The molar mass is 130.10 g · mol −1 with a density of 1.63 g · cm −3 . It is flammable and hygroscopic as well as moderately soluble in water (83 g · l −1 at 20 ° C).
Extraction and presentation
The technical production of itaconic acid takes place biotechnologically through the submerged fermentation of molasses and other substrates with strains of the fungi Aspergillus itaconicus or Aspergillus terreus . In theory, when a 15% sucrose solution is used, 78% is converted to itaconic acid. The sugar-based production volume is more than 80 g / l.
The metabolic pathway was first demonstrated in 1931 for Aspergillus niger (today Aspergillus itaconicus ) and shortly thereafter also for Aspergillus terreus . Itaconic acid is formed as a by-product of the citrate cycle when citric acid (citrate) is converted to isocitric acid (isocitrate) via the cis -aconitic acid . The cis-aconitic acid is decarboxylated to produce itaconic acid ( i.e. CO 2 is removed) instead of being hydrated to isocitric acid through the addition of water . The cis-aconitic acid decarboxylase is used as the enzyme for the conversion to itaconic acid . The corn bite brandy Ustilago maydis uses an alternative biosynthetic pathway. cis -aconitic acid is converted to the thermodynamically favored trans -aconitic acid with the aid of aconitate Δ isomerase (Adi1) . The trans -aconitic acid is decarboxylated to itaconic acid analogously to Aspergillus by the trans -aconitate decarboxylase (Tad1).
Another alternative biosynthetic pathway runs from pyruvic acid (pyruvate) via citrate malic acid , citraconic acid and itatartaric acid to itaconic acid.
Succinic acid and itartaric acid, which are undesirable in technical processes, are produced as by-products . The formation can be prevented by adding calcium , which inhibits itaconic acid oxidase .
use
The total amount of the worldwide production of itaconic acid amounts to more than 80,000 tons per year at a market price of about US $ 2 / kg (as of 2009).
Itaconic acid is used as a comonomer for the synthesis of polyacrylates and rubber . It is also used in the manufacture of paints and varnishes, as a thickener for fats, for pharmaceuticals, as a herbicide and for biodegradable polymers in the packaging industry.
Together with other biotechnologically relevant chemicals, itaconic acid was identified in 2004 by the US Department of Energy as one of twelve platform chemicals with particular biotechnological manufacturing potential. After a renewed assessment, however, it was removed from the list of hopefuls in 2010 due to various criteria.
Individual evidence
- ↑ a b c d e f g h Entry on propene-2,3-dicarboxylic acid in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ T. Willke, K.-D. Vorlop: Biotechnological production of itaconic acid. In: Applied Microbiology and Biotechnology . 56 (3), Aug 2001, pp. 289-295. doi: 10.1007 / s002530100685 .
- ↑ a b OECD: Data sheet Butanedioic acid, methylene (English).
- ↑ a b c Garabed Antranikian: Applied Microbiology. Springer-Verlag, Berlin / Heidelberg 2006, ISBN 3-540-24083-7 , pp. 351-352.
- ↑ a b M. Okabe, D. Lies, S. Kanamasa, EY Park: Biotechnological Production of Itaconic Acid and its Biosynthesis from Aspergillus terreus. In: Applied Microbiology and Biotechnology . 84 (4), September 2009, pp. 597-606.
- ↑ Elena Geiser, Sandra K Przybilla, Alexandra Friedrich, Wolfgang Buckel, Nick Wierckx, Lars M Blank, Michael Bölker: Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate . In: Microbial Biotechnology . tape 9 , no. 1 , January 1, 2016, ISSN 1751-7915 , p. 116-126 , doi : 10.1111 / 1751-7915.12329 , PMID 26639528 , PMC 4720413 (free full text).
- ↑ T. Werpy, G. Petersen: Top Value Added Chemicals from Biomass. Volume I - Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Produced by the Staff at Pacific Northwest National Laboratory (PNNL); National Renewable Energy Laboratory (NREL), Office of Biomass Program (EERE), 2004 (PDF) .
- ^ Joseph J. Bozell, Gene R. Petersen: Technology development for the production of biobased products from biorefinery carbohydrates - the US Department of Energy's 'Top 10' revisited. In: Green Chemistry . 12, 2010, pp. 539-554.
