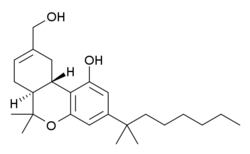
Dexanabinol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Dexanabinol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 25 H 38 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 386,57 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Dexanabinol (HU-211) is a synthetic cannabinoid that does not bind to CB 1 and CB 2 receptors . It is a research chemical that is currently not approved for marketing.
chemistry
Dexanabinol is the enantiomer of HU-210 , a very potent cannabinoid. Structurally, it is the mirror image of the side chain homologue of the THC metabolite 11-hydroxy-Δ 9 -tetrahydrocannabinol.
The original synthesis of HU-211 is based on an acid-catalyzed condensation of (+) - myrthenol and 1,1-dimethylheptylresorcinol (3,5-dihydroxy-1- (1,1-dimethylheptyl) benzene).
It was developed in Raphael Mechoulam's group at the Hebrew University in Jerusalem. HU-211 does not bind to known cannabinoid receptors but to NMDA receptor channels and has therefore been tested as a potential drug for traumatic brain injury , but was no more effective than placebo in clinical studies.
literature
- K. Razdan: The Total Synthesis of Cannabinoids . In: John Apsimon (Ed.): The Total Synthesis of Natural Products . Wiley Interscience, 1981, ISBN 978-0-471-05460-3 , p. 245.
Web links
- Material Safety Data Sheet HU-211 (PDF; 19 kB)
- HU-211 as a product from Cayman Chemical
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A label of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 14, 2020, is derived from a self-classification by the distributor .
- ↑ R. Mechoulam, N. Lander, A. Breuer, J. Zahalka: Synthesis of the Individual, Pharmacologically Distinct, Enantiomers of a Tetrahydrocannabinol Derivative . In: Tetrahedron: Asymmetry , 1990, 5, pp. 315-318.
- ↑ R Zeltser, Z Seltzer, A Eisen, JJ Feigenbaum, R Mechoulam: Suppression of neuropathic pain behavior in rats by a non-psychotropic synthetic cannabinoid with NMDA receptor-blocking properties . In: Pain . 47, No. 1, October 1991, pp. 95-103. PMID 1663228 .
- ^ AI Maas, G Murray, H Henney et al .: Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomized, placebo-controlled, clinical trial . In: The Lancet Neurology . 5, No. 1, January 2006, pp. 38-45. doi : 10.1016 / S1474-4422 (05) 70253-2 . PMID 16361021 .
