Anandamide
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
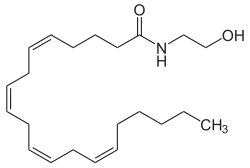
|
|||||||||||||
| General | |||||||||||||
| Surname | Anandamide | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 22 H 37 NO 2 | ||||||||||||
| Brief description |
light yellow, oily liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 347.53 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
0.92 g cm −3 |
||||||||||||
| solubility |
soluble in ethanol |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Arachidonylethanolamide , also anandamide called, which is ethanolamine - derivative of arachidonic acid , a four-fold unsaturated fatty acid , which is particularly frequent in the central nervous system occurs. Anandamide is produced by the body itself (if linoleic acid is present) and is therefore an endogenous substance.
effect
Anandamide (from Sanskrit Ananda : joy or pure happiness) binds to the receptors of the endocannabinoid system to which the THC of the cannabis plant docks. With sufficient dosage, it is also able to displace THC and other cannabinoids. It also binds to vanilloid TRPV1 receptors.
The main interacting regions of the brain seem to be those that are concerned with perception and thought processing or movement processes.
Anandamide can be metabolized by cyclooxygenase-2 (but not by cyclooxygenase-1 ) to prostanoids, the function of which is still unknown. However, arachidonylethanolamide is largely broken down by fatty acid amide hydrolase (FAAH) by splitting into arachidonic acid and ethanolamine.
In March 2008, British scientists from the University of Leicester published an article in the Journal of the American Medical Association in which they speculated that increased anandamide levels in the blood of pregnant women could be a sign of an increased risk of miscarriage.
structure
In terms of structural chemistry, there are significant differences between anandamide and THC. The pronounced lipophilicity (fat solubility), however, is common to both compounds. However, detailed investigations showed that THC and anandamide have a very similar three-dimensional structure. Pharmacokinetically , anandamide is broken down much faster (after approx. 30 minutes no more measurable effect) than THC, which remains effective for a few hours.
Occurrence
Anandamide and the ethanolamides of two other unsaturated fatty acids (oleoyl and linoleoylethanolamide) have been detected in chocolate and cocoa powder .
discovery
Anandamide was discovered in 1992 by the Czech chemist Lumír Ondřej Hanuš and the American molecular pharmacologist William Anthony Devane; shortly thereafter, its pharmacological effects were described.
literature
- R. Mechoulam, E. Fride: The unpaved road to the endogenous brain cannabinoid ligands, the anandamides. In: R. Pertwee (Ed.): Cannabinoid Receptors. Academic Press, London 1995, ISBN 0-12-551460-3 , pp. 233-258.
Individual evidence
- ↑ a b c d e data sheet arachidonylethanolamide from Sigma-Aldrich , accessed on June 16, 2011 ( PDF ).
- ^ DocCheck Medical Services GmbH: Arachidonic acid - DocCheck Flexikon. Retrieved August 15, 2018 .
- ^ RA Ross: Anandamide and vanilloid TRPV1 receptors . In: Br. J. Pharmacol. tape 140 , no. 5 , 2003, p. 790-801 , doi : 10.1038 / sj.bjp.0705467 , PMID 14517174 .
- ↑ Osama MH Habayeb, Anthony H. Taylor, Mark Finney, Mark D. Evans, Justin C. Konje: Plasma Anandamide Concentration and Pregnancy Outcome in Women With Threatened Miscarriage . In: Journal of the American Medical Association . tape 299 , no. 10 , 2008, p. 1135-1136 .
- ↑ J. Barnett-Norris, DP Hurst, DL Lynch, F. Guarnieri, A. Makriyannis, PH Reggio: Conformational memories and the endocannabinoid binding site at the cannabinoid CB1 receptor . In: J. Med. Chem. Volume 45 , no. 17 , 2002, p. 3649-3659 , PMID 12166938 .
- ^ E. di Tomaso, M. Beltramo, D. Piomelli: Brain cannabinoids in chocolate . In: Nature . tape 382 , no. 6593 , 1996, pp. 677-678 , doi : 10.1038 / 382677a0 , PMID 8751435 .
- ↑ WA Devane, L. Hanus, A. Breuer et al: Isolation and structure of a brain constituent that binds to the cannabinoid receptor . In: Science . tape 258 , no. 5090 , 1992, pp. 1946-1949 , PMID 1470919 .