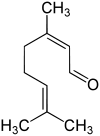
Citral
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| left Geranial (Citral A), right Neral (Citral B) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Citral | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 16 O | |||||||||||||||
| Brief description |
light yellow liquid with a lemon-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 152.24 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.89 g cm −3 |
|||||||||||||||
| Melting point |
<−20 ° C |
|||||||||||||||
| boiling point |
225 ° C |
|||||||||||||||
| Vapor pressure |
0.046 h Pa (20 ° C) |
|||||||||||||||
| solubility |
almost insoluble in water (420 mg l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.4898 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Citral is the mixture of the cis - trans isomers geranial (Citral A) and neral (Citral B). Geranial is used as a perfume u. a. to be found in tomatoes in small proportions as a breakdown product of lycopene . Citral is the main component of lemongrass oil . It is the alarm pheromone of the leaf cutter ant .
Extraction and presentation
In addition to being extracted from Litsea cubeba oil (content 60 to 80%), citral can also be obtained through various chemical syntheses. For example, through the reaction of isobutylene with formaldehyde to form isoprenol and its reaction to isoprenal with the help of a silver catalyst and then subsequent reactions with prenol to form citral. Other known syntheses start from isoprene or pinenes or are carried out by pyrolysis of limonene .
properties
Citral and neral and geranial include chemically to the group of acyclic mono terpene - aldehydes . Citral is a pale yellowish liquid with an intensely fresh lemon scent. The mixture boils at 228 ° C and is almost insoluble in water. Citral is irritating to the skin in its pure form and in a mixture in concentrations of 1% or more.
use
Citral is used as a fragrance and aroma. According to the 7th amendment to the EU Cosmetics Directive 76/768 / EEC, it must be declared as a cosmetic ingredient due to its allergenic potential.
Reactions
Neral and geranial can be made from nerol and geraniol by dehydration . Citral reacts with acetone in an alkaline environment (for example barium hydroxide ), initially releasing water to form pseudoionone . This can then be converted into the isomer ionone in the presence of acids and at higher temperatures .
In an alkaline environment it can also break down into acetaldehyde and 2-methylhept-2-en-6-one (retro- aldol reaction ).
The heterogeneously catalyzed hydrogenation in the presence of palladium catalysts leads via the intermediate citronellal to dihydrocitronellal with about 86% yield .
By reaction with hydroxylamine to Citraloxim and its dehydration can geranylnitrile be won.
Risk assessment
In 2014, citral was included in the EU's ongoing action plan ( CoRAP ) in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the inclusion of citral were concerns about consumer use , exposure of workers , high (aggregated) tonnage and widespread use as well as the suspected hazards from sensitizing properties. The re-evaluation took place from 2015 and was carried out by Sweden . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i Entry on citral in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-118.
- ↑ Entry on citral in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ E. Boyland: Experiments on the chemotherapy of cancer: Further experiments with aldehydes and their derivatives. In: The Biochemical journal. Volume 34, Numbers 8-9, September 1940, pp. 1196-1201, PMID 16747303 , PMC 1265400 (free full text).
- ↑ a b Entry on citral in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on February 2, 2018.
- ^ PM Jenner, EC Hagan, Jean M. Taylor, EL Cook, OG Fitzhugh: Food flavors and compounds of related structure I. Acute oral toxicity. In: Food and Cosmetics Toxicology. 2, 1964, pp. 327-343, doi : 10.1016 / S0015-6264 (64) 80192-9 .
- ^ Ralf Günter Berger: Flavors and Fragrances Chemistry, Bioprocessing and Sustainability . Springer Science & Business Media, 2007, ISBN 978-3-540-49339-6 , pp. 288 ( limited preview in Google Book search).
- ↑ Volker Hessel: Design and Engineering of Microreactor and Smart-Scaled Flow Processes . MDPI, 2018, ISBN 978-3-03842-038-5 , pp. 128 ( limited preview in Google Book search).
- ^ Charles S. Sell: A Fragrant Introduction to Terpenoid Chemistry . Royal Society of Chemistry, 2007, ISBN 978-1-84755-001-9 , pp. 295 ( limited preview in Google Book search).
- ↑ Juliane Daphi-Weber, Heike Raddatz, Rainer Müller: Investigation of Fragrances - Controlled Fragrances , pp. 94–95, in Volume V of the series HighChem hautnah - News from food chemistry (published by the Society of German Chemists ) 2010, ISBN 978- 3-936028-64-5 .
- ↑ NODA, C., ALT, GP, WERNECK, RM et al .: Aldol Condensation of Citral with Acetone on Basic Solid Catalysts, in: Braz. J. Chem. Eng. , 1998 , 15 ; doi : 10.1590 / S0104-66321998000200004 ; Abstract .
- ^ A. Russell, RL Kenyon: Pseudoionone In: Organic Syntheses . 23, 1943, p. 78, doi : 10.15227 / orgsyn.023.0078 ; Coll. Vol. 3, 1955, p. 747 ( PDF ).
- ↑ P. Claus, J. Arras, D. Ruppert: Influence of ionic liquids with functionalized cations on the palladium-catalyzed liquid-phase hydrogenation of citral , in: Chem. Ing. Techn. 81 (2009), pp. 2007-2011; doi : 10.1002 / cite.200900085 .
- ^ Charles Sell: The Chemistry of Fragrances From Perfumer to Consumer . Royal Society of Chemistry, 2006, ISBN 978-0-85404-824-3 , pp. 67 ( limited preview in Google Book search).
- ↑ European Chemicals Agency (ECHA): Substance Evaluation Conclusion and Evaluation Report .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Citral , accessed on March 26, 2019.
