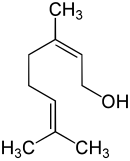
Nerol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Nerol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 18 O | |||||||||||||||
| Brief description |
colorless, liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 154.25 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.88 g cm −3 |
|||||||||||||||
| Melting point |
−10 ° C |
|||||||||||||||
| boiling point |
224-225 ° C |
|||||||||||||||
| Refractive index |
1.474 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Nerol (according to IUPAC ( Z -2,6-dimethyl-2,6-octadien-8-ol) called) is an acyclic monoterpene - alcohol . The clear liquid has a fresh, sweet, rosy-citrus-like odor. Nerol is isomeric to geraniol .
structure
Geraniol is the trans isomer, nerol the cis isomer of the compound with the same empirical formula. It is a primary, monohydric alcohol: the molecule has a hydroxyl group , only one additional carbon atom is directly bonded to the carbon atom to which this hydroxyl group is attached.
Occurrence
Nerol is found in many essential oils such as lavender or rose oil . It always occurs together with geraniol.
Manufacturing
Nerol is a by-product of the production of geraniol and is mainly obtained in this way.
properties
The flash point is 100 ° C. Less than a gram of nerol is soluble in one liter of water . At the air nerol will nerol oxide oxidized . Nerol is harmful to health.
use
It is required for the production of citral , citronellol , vitamin A and vitamin E. Nerol is used as a fragrance in perfumery in rose and flower fragrance compositions.
Individual evidence
- ↑ a b Nerol data sheet (PDF) from Carl Roth , accessed on December 14, 2010.
- ↑ a b Nerol data sheet at AlfaAesar, accessed on December 14, 2010 ( PDF )(JavaScript required) .
- ↑ Entry on Geraniole. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ Nerol data sheet from Sigma-Aldrich , accessed on April 15, 2011 ( PDF ).
- ↑ a b entry to nerol in GESTIS Bank of IFA , accessed on July 23, 2016(JavaScript required) .
- ^ A b Horst Surburg & Johannes Panten: Common Fragrance and Flavor Materials . 6. Completely revised and expanded edition. Wiley-VCH, 2016, ISBN 978-3-527-33160-4 , pp. 32 .

