Androsterone
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
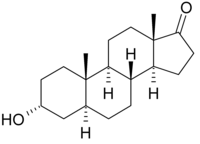
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Androsterone | ||||||||||||||||||
| other names |
3 α - hydroxy- 5 α -androstan-17-one |
||||||||||||||||||
| Molecular formula | C 19 H 30 O 2 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 290.44 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
185-185.5 ° C |
||||||||||||||||||
| solubility |
Hardly soluble in water, soluble in most organic solvents |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Androsterone (ADT) is an androgen (male sex hormone ). It is a metabolite of the sex hormone testosterone with weak androgenic activity and is formed in the liver . Androsterone, like testosterone, regulates the sexual drive and is important for the development of male secondary sexual characteristics.
Androsterone was the first isolated steroid hormone . It was found in male urine in 1931 by Adolf Butenandt and Kurt Tscherning . To do this, they distilled 25,000 liters and extracted 50 milligrams of crystalline androsterone from it.
Web links
Wiktionary: Androsterone - explanations of meanings, word origins, synonyms, translations
Individual evidence
- ↑ a b The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, pp. 103-104, ISBN 978-0-911910-00-1 .
- ↑ a b data sheet androsterone from Sigma-Aldrich , accessed on March 9, 2011 ( PDF ).
- ↑ Butenandt, A. & Tscherning, K. (1934): About Androsteron, a crystallized male sex hormone. I Isolation and purification from male urine. In: Z. Physiol. Chem. Vol. 229, pp. 167-184.