Glycine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
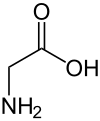
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Glycine | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 2 H 5 NO 2 | |||||||||||||||||||||
| Brief description |
colorless and odorless, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 75.07 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density |
1.161 g cm −3 |
|||||||||||||||||||||
| Melting point |
Decomposition: 232-236 ° C |
|||||||||||||||||||||
| pK s value |
|
|||||||||||||||||||||
| solubility |
easily soluble in water
|
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| Thermodynamic properties | ||||||||||||||||||||||
| ΔH f 0 |
−528.5 kJ / mol |
|||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Glycine , abbreviated to Gly or G , (also glycine or glycocolla , from ancient Greek κόλλα kólla: glue, according to systematic chemical nomenclature aminoacetic acid or aminoethanoic acid), is the smallest and simplest α - amino acid and was first obtained in 1820 from gelatin, i.e. from collagen hydrolyzate . It belongs to the group of hydrophilic amino acids and is the only proteinogenic (or protein-forming) amino acid achiral and therefore not optically active .
Glycine is not essential, so it can be synthesized by the human organism itself and is an important component of almost all proteins and an important node in the metabolism.
The name is derived from the sweet taste of pure glycine ( Greek γλυκύς glykýs , German 'sweet' ).
history
Glycine is the first amino acid that was obtained by acidic digestion of proteins. The director of the Botanical Garden in Nancy, Henri Braconnot , succeeded in isolating glycine after purification by digesting animal glue with sulfuric acid . These were sweet-tasting crystals. Assuming a sugar, he called the substance “sucre de gélatine” ( gelatine is the main component of glutinous glue ), in English glue sugar, according to its origin . Soon afterwards the substance was renamed glycocolla (“sweet glue”) before Jöns Jakob Berzelius decided in 1848 that he would use the shorter name glycine from now on. The chemical structure was not correctly described until 1858 by the French chemist Auguste André Thomas Cahours .
synthesis
The aminonitrile (more precisely: α- aminoacetonitrile ) formed during the reaction of formaldehyde , hydrogen cyanide and ammonia ( Strecker synthesis ) yields glycine during hydrolysis :
As a partial reaction, this reaction played a special role in the hypothesis that organic molecules as “building blocks” for the first primitive organisms were created around 4 billion years ago from the simple inorganic compounds of the primordial atmosphere of the earth. A composition of water (H 2 O), methane (CH 4 ), ammonia (NH 3 ), hydrogen (H 2 ) and carbon monoxide (CO) as well as helium (He) and other noble gases was assumed for this primordial atmosphere (cf. → Miller -Urey experiment ).
Chemically, glycine can also be produced from monochloroacetic acid and ammonia:
Most of the glycine in the body is absorbed through food, but it can also be made from serine .
properties
Glycine is mainly present as an "inner salt" or zwitterion , the formation of which can be explained by the fact that the proton of the acidic carboxy group migrates to the lone pair of electrons on the nitrogen atom of the basic amino group :
The zwitterion does not migrate in the electric field because it is uncharged as a whole. Strictly speaking, this is the case at the isoelectric point (at a certain pH value, here 5.97), at which the glycine also has its lowest solubility in water.
- Van der Waals volume : 48
- Degree of hydrophobicity : −0.4
Free glycine has a sweet taste, wherein the detection threshold value at 25 to 35 mmol / L is located.
Occurrence
The following examples give an overview of the glycine content and each relate to 100 g of the food; the percentage of glycine in relation to the total protein is also given:
| Food | Total protein |
Glycine | proportion of |
|---|---|---|---|
| Pork , raw | 21 g | 0.95 g | 4.5% |
| Chicken breast fillet , raw | 21 g | 0.95 g | 4.4% |
| Salmon , raw | 20.5 g | 0.95 g | 4.7% |
| Gelatin powder , unsweetened | 86 g | 19 g | 22.3% |
| Chicken egg | 12.5 g | 0.43 g | 3.4% |
| Cow's milk , 3.7% fat | 3.3 g | 0.07 g | 2.1% |
| Walnuts | 15 g | 0.82 g | 5.4% |
| Pumpkin seeds | 30 g | 1.85 g | 6.1% |
| Wholemeal wheat flour | 14 g | 0.55 g | 4.0% |
| Wholemeal corn flour | 7.0 g | 0.28 g | 4.1% |
| Rice , unpeeled | 8.0 g | 0.39 g | 4.9% |
| Soybeans , dried | 36.5 g | 1.9 g | 5.2% |
| Peas , dried | 24.5 g | 1.1 g | 4.4% |
All of these foods contain almost exclusively chemically bound glycine as a protein component, but no free glycine.
In 2009, glycine was detected in the cometary dust of Wild 2 ; In 2016 it was also detected in the gas cloud of comet 67P / Churyumov-Gerasimenko .
Functions
metabolism
The conversion of serine to glycine is used not only to generate glycine but also to convert tetrahydrofolic acid into N 5 -N 10 -methylene tetrahydrofolic acid (TH4), which is required, among other things, for the synthesis of thymine nucleotides ( DNA component).
Conversely, glycine can be used to synthesize serine by absorbing CH 3 from TH4, which is then available for protein synthesis, as the basic substance of choline or as pyruvate.
Glycine is also often required for the synthesis of other components of the genetic material ( purines ).
It is also used for the biosynthesis of heme ( oxygen binding in the blood ), creatine (energy store in the muscle) or glutathione :
- Glycine + succinyl-CoA → 5-aminolevulinic acid → porphyrin synthesis to build up the heme.
- Glycine + guanodine group (from arginine ) → guanidinoacetate , which can then be used in creatinine synthesis .
- Glycine + Glu-Cys peptide bond → glutathionic acid
Oxalic acid , which is harmful to health, can also be formed from glycine as a by-product .
As a so-called glucogenic or glucoplastic amino acid, glycine can be converted into glucose via pyruvate in the course of metabolism .
Protein component
Due to its small size, glycine is preferably incorporated into polypeptides in spatially restricted positions (the protein secondary structure ).
It is particularly common in collagen , the most common protein in animal organisms. Here it makes up a good third of all amino acids, because due to its small size it allows the collagen to be rolled up to form its triple helix structure .
Nervous system
Glycine acts in the central nervous system via the glycine receptor as an inhibitory neurotransmitter , i.e. as an inhibiting signal substance. The action takes place via the opening of ligand-controlled chloride channels and thus leads to an inhibitory postsynaptic potential (IPSP), which reduces the activity of the downstream nerve cell.
At the NMDA receptor, on the other hand, in addition to the main agonist glutamate , it has a stimulating effect on a special glycine binding site.
Glycine-releasing nerve cells (glycinergic neurons) are found mainly in the brain stem and spinal cord , in the latter they inhibit the activity of the motor neurons of the anterior horn , which leads to a reduction in the activity of the muscles innervated by these cells.
Strychnine , which acts as an antagonist and blocks the binding site of the glycine receptor, and tetanus toxin , which inhibits the release of glycine, reduce the effect of glycine. By blocking the glycine receptors or reducing the glycine level, the inhibition of motor neuron activity is reduced, so that life-threatening cramps can occur.
Glycine encephalopathy can result from abnormal accumulation of glycine .
use
Glycine is added to foods as a flavor enhancer .
Glycine and its sodium salt are generally permitted in the EU as a food additive with the number E 640 without maximum amount restrictions for food, negative health effects are not known.
Glycine is also a component of infusion solutions for parenteral nutrition.
In monopolar transurethral resection , glycine can be used as an additive to the irrigation fluid in addition to a mixture of mannitol and sorbitol .
In molecular biological and biochemical research, glycine is used in the form of a TRIS -Glycine buffer system for protein separation using SDS-PAGE ; the glycine ions act as secondary ions in the stacking gel.
literature
- G. Löffler, PE Petrides: Biochemistry and Pathobiochemistry. 7th edition. Springer Verlag, 2003, ISBN 3-540-42295-1 .
Web links
Individual evidence
- ↑ entry to GLYCINE in CosIng database of the European Commission, called on July 8 2020th
- ↑ Entry on E 640: Glycine and its sodium salt in the European database for food additives, accessed on August 11, 2020.
- ↑ a b c d e f Entry on glycine in the GESTIS substance database of the IFA , accessed on December 17, 2019(JavaScript required) .
- ^ A b F.A. Carey: Organic Chemistry. 5th Edition The McGraw Companies, 2001, p. 1059, Link
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: Amino acids, peptides, proteins , Verlag Chemie, Weinheim, pp. 38–43, 1982, ISBN 3-527-25892-2 .
- ↑ a b c d e Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . 1. Student Edition. CRC Press, Boca Raton, Florida 1988, ISBN 0-8493-0740-6 , pp. C-706.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-22.
- ^ H. Braconnot, Sur la Conversion des matières animales en nouvelles substances par le moyen de l`acide sulfurique., Ann. Chim. Phys., Volume 10, pp. 29ff (1819).
- ↑ S. Hansen: The discovery of proteinogenic amino acids from 1805 in Paris to 1935 in Illinois. ( Memento of the original from June 15, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Berlin 2015.
- ↑ PM Hardy: The Protein Amino Acids. In: GC Barrett (Ed.): Chemistry and Biochemistry of the Amino Acids. Chapman and Hall, 1985, ISBN 0-412-23410-6 , p. 9.
- ↑ W. Ternes, A. Täufel, L. Tunger, M. Zobel (eds.): Food Lexicon. 4th edition. Behr's Verlag, Hamburg 2005, ISBN 3-89947-165-2 , p. 62f.
- ↑ nutrient database of the US Department of Agriculture , 22nd edition.
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, p. 19, 1982, ISBN 3-527-25892-2 .
- ↑ NASA Researchers Make First Discovery of Life's Building Block in Comet nasa.gov, August 2009; Building blocks of life from all around Spektrum.de, August 2009 (accessed October 4, 2010).
- ↑ Jamie E. Elsila, et al .: Cometary glycine detected in samples returned by Stardust. Meteoritics & Planetary Science 44, No. 9, 1323-1330 (2009), pdf online @ gsfc.nasa.gov, accessed November 23, 2011.
- ↑ Wolfgang Stieler: Amino acid found in comets. Heise.de, Technology Review, May 27, 2016.
- ^ Georg Löffler, Petro E. Petrides, Peter C. Heinrich: Biochemistry & Pathobiochemistry. Springer Medizin Verlag, Heidelberg 2006, ISBN 3-540-32680-4 , p. 1040.
- ^ S. Ebel, HJ Roth (Ed.): Lexicon of Pharmacy. Georg Thieme Verlag , 1987, ISBN 3-13-672201-9 , p. 28.
- ^ Dawkins GPC and Miller R. A Sorbitol-Mannitol Solution for Urological Electrosurgical Resection - A safer Fluid than Glycine 1.5% European Urology 1999; 36: 99-102.
- ^ UK Laemmli: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. In: Nature . Vol. 227, 1970, pp. 680-685, doi: 10.1038 / 227680a0 , PMID 5432063 .







