5-aminolevulinic acid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | 5-aminolevulinic acid | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 5 H 9 NO 3 | |||||||||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 131.13 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility |
good in water (50 g l −1 , hydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
5-aminolevulinic acid (5-ALA) is an amino acid from the group of ketocarboxylic acids . It is approved as gel Ameluz and as drug plaster Alacare for the treatment of actinic keratoses by means of photodynamic therapy (PDT) and as gliolan for photodiagnostics (FD).
Extraction and presentation
In addition to chemical processes, 5-aminolevulinic acid can also be produced by biotechnological processes with the help of bacteria (Rhodobacterium, Propionibacterium, Methanobacterium, Methanosarcina and the like).
Biological importance
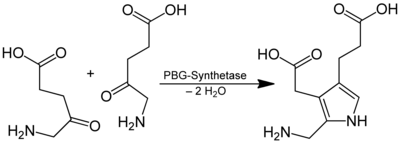
5-aminolevulinic acid is a precursor of heme in porphyrin synthesis . Two molecules react to form porphobilinogen , splitting off water :
The δ-aminolevulinate ion is formed in a reaction catalyzed by the mitochondrial enzyme δ-aminolevulinate synthase from succinyl-CoA and the amino acid glycine :
- Succinyl-CoA + glycine → δ-aminolevulinate + CO 2 + CoA
 δ-aminolevulinate ( zwitterion )
δ-aminolevulinate ( zwitterion )
The required succinyl-CoA comes from the citric acid cycle , glycine from the biosynthesis from serine . In the further course of the heme biosynthesis, two molecules of δ-aminolevulinate react to form the so-called porphobilinogen (PBG) and two water molecules . This reaction is catalyzed by the enzyme δ-aminolevulinic acid dehydratase (synonym: porphobilinogen synthase) .
- 2 δ-aminolevulinate → PBG + 2 H 2 O
The resulting porphobilinogen is then transported from the mitochondrion into the cytosol . Protoporphyrin IX, which is important for photodynamic therapy, is formed here from four molecules of porphobilinogen via three further intermediate stages .
Pharmacological effect
5-ALA accumulates more in tumor cells and tumor-like cells than in healthy cells, where it is converted to protoporphyrin IX. The resulting increased accumulation of the red fluorescent molecule protoporphyrin IX in these cells is used in tumor diagnostics and photodynamic therapy (PDT). In addition to the increased accumulation of 5-ALA in tumor cells, the synthesis of the photosensitizer protoporphyrin IX from 5-ALA is favored compared to healthy cells, since certain metabolic precursors of protoporphyrin IX, porphobilinogen and uroporphyrinogen III, are formed to a greater extent. In contrast, the breakdown of protoporphyrin IX to heme is reduced by a lower activity of ferrochelatase in tumor cells than in healthy cells. The external addition of 5-ALA as a drug also circumvents the end product inhibition of heme on the 5-ALA synthase of the cell. Protoporphyrin IX is more efficiently synthesized from 5-ALA in various cell types than from its methyl ester (MAOP). In a study on skin cells, it was shown that protoporphyrin IX from 5-ALA accumulated four times more strongly in skin tumor cells than in healthy cells after an exposure time relevant for photodynamic therapy. The accumulation from 5-ALA in the tumor cells was almost twice as high as that from MAOP, but in healthy cells it was equally low for both active substances. The increased accumulation of protoporphyrin IX in tumor cells is used in photodynamic therapy to selectively kill them. By exposure to suitable light sources, Protoporphyrin IX absorbs light energy and transfers it to oxygen , which becomes very reactive, toxic singlet oxygen . This specifically destroys the tumor tissue in which protoporphyrin IX was preferentially formed, whereas healthy cells are largely spared.
use
Medical
Brain tumors
The intracellular accumulation in the malignant brain tumor glioblastoma and conversion to protoporphyrin IX there enables better intraoperative delimitation of the tumor margin from the adjacent brain tissue and thereby facilitates tumor resection. To do this, 5-ALA is taken about 2 to 4 hours before the operation. Because of possible skin damage through photosensitivity, patients should not be exposed to strong light sources (e.g. operating theater lighting, direct sunlight or bright, intensive interior lighting) for 24 hours.
Skin tumors
Externally, 5-ALA is indicated as part of the photodynamic treatment of mild to moderate actinic keratosis , a common precancerous condition caused by intensive exposure to UV radiation . Photodynamic therapy is the most successful treatment available for actinic keratosis and is therefore recommended as the first choice of therapy in the dermatological guidelines. For the treatment of actinic keratosis, 5-ALA is approved as a medicinal plaster or gel. The nanoemulsion contained in the gel significantly improves the low stability and skin penetration of 5-ALA compared to conventional emulsions. The drug is applied to the lesions on the skin and the treated area is illuminated with a cold red light source after 3 to 4 hours of exposure for 10 to 20 minutes.
In most patients (up to 80%) the side effects of photodynamic therapy with 5-ALA are local skin irritations in the form of erythema and edema as well as pain during and shortly after the treatment. In the days after the treatment, there is usually a short-term, sterile and therefore harmless inflammation of the treated skin, which probably contributes significantly to the success of the treatment. In the meantime, the skin undergoes regeneration processes and an increased formation of collagen, which is partly responsible for the good cosmetic result of the therapy. PDT does not leave any scars. In a direct comparison, a significantly higher healing success for photodynamic therapy with 5-ALA compared to that with MAOP was documented, with the same frequency and intensity of the side effects caused by the treatment such as redness and pain. After an observation period of 12 months, there was a lower relapse rate for patients who received PDT with 5-ALA compared to patients who were treated with MAOP.
In the case of acute lead poisoning , an increased level of 5-aminolevulinic acid in the urine is a sensitive detection method.
Traditionally, 5-ALA has been widely used in formulations. However, according to the more recent legal situation, this is legally questionable and entails possible liability risks for doctors and pharmacists. Approved finished medicinal products with 5-ALA with improved stability and scientifically proven high efficacy are available. In the New Formulation Form (NRF) of the Federal Association of German Pharmacists' Associations (ABDA) there is no monograph on the production of ALA formulations due to the high instability of 5-ALA in conventional formulation bases. According to a resolution of the Council of Ministers of the European Union of January 19, 2011, the equivalent, approved finished medicinal product must be used instead of a formulation if there is no clinically or scientifically justifiable additional benefit of the formulation. Therefore, warnings against the use of ALA formulations are increasing.
Agricultural
5-aminolevulinic acid is used as a fertilizer in agriculture.
Finished medicinal products
Alacare (D), Ameluz (EU), Gliolan (EU)
Web links
- Cubic gel and plaster: Competing dosage forms for 5-aminolevulinic acid (PDF; 35 kB)
- Daniela Steinat: Evaluation of the photodynamic diagnosis with 5-aminolevulinic acid in basal cell carcinoma by 3-D histology. urn : nbn: de: bsz: 21-opus-15597
- Publicly accessible approval data for Ameluz® European Medical Association (EMA)
Individual evidence
- ↑ a b c d Data sheet 5-Aminolevulinic acid hydrochloride from Sigma-Aldrich , accessed on February 5, 2018 ( PDF ).
- ↑ a b Entry on 5-amino-4-oxopentanoic acid. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ a b c d Ameluz 78 mg / g gel, Summary of Product Characteristics, as of March 2013.
- ↑ a b c d Specialist information Alacare 8 mg plaster containing active ingredients, as of February 2011.
- ↑ a b Technical information Gliolan 30 mg / ml (PDF; 93 kB) as of July 2012.
- ↑ Microorganisms that produce 5-aminolevulinate and methods for producing 5-aminolevulinate using the same . Patent de
- ↑ a b R Van Hillegersberg, JW Van den Berg, WJ Kort, OT Terpstra, JH. Wilson: Selective accumulation of endogenously produced porphyrins in a liver metastasis model in rats . In: Gastroenterology . 1992 Aug, 103 (2), pp. 647-651, PMID 1386052 .
- ↑ M Kondo, N Hirota, T Takaoka, M. Kajiwara: Heme-biosynthetic enzyme activities and porphyrin accumulation in normal liver and hepatoma cell lines of rat . In: Cell Biol Toxicol . , 1993 Jan-Mar, 9 (1), pp. 95-105, PMID 8390914 .
- ↑ L Leibovici, N Schoenfeld, HA Yehoshua, R Mamet, E Rakowsky, A Shindel, A. Atsmon: Activity of porphobilinogen deaminase in peripheral blood mononuclear cells of patients with metastatic cancer . In: Cancer . 1988 Dec 1, 62 (11), pp. 2297-2300, PMID 3179945 .
- ↑ N Schoenfeld, O Epstein, M Lahav, R Mamet, M Shaklai, A. Atsmon: The heme biosynthetic pathway in lymphocytes of patients with malignant lymphoproliferative disorders . In: Cancer Lett . , 1988 Dec 1, 43 (1-2), pp. 43-48, PMID 3203329 .
- ↑ MM el-Sharabasy, AM el-Waseef, MM Hafez, SA. Salim: Porphyrin metabolism in some malignant diseases . In: Br J Cancer . 1992 Mar, 65 (3), pp. 409-412, PMID 1558795 .
- ↑ a b Q Peng, K Berg, J Moan, M Kongshaug, JM. Nesland: 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research . In: Photochem Photobiol. , 1997 Feb, 65 (2), pp. 235-251, PMID 9066303 .
- ↑ MA. MacCormack: Photodynamic therapy in dermatology: an update on applications and outcomes . In: Semin Cutan Med Surg. 2008 Mar, 27 (1), pp. 52-62, PMID 18486025 .
- ^ R Washbrook, H Fukuda, A Battle, P. Riley: Stimulation of tetrapyrrole synthesis in mammalian epithelial cells in culture by exposure to aminolaevulinic acid . In: Br J Cancer. 1997, 75 (3), pp. 381-387, PMID 9020483 .
- ^ P Uehlinger, M Zellweger, G Wagnières, L Juillerat-Jeanneret H, van den Bergh, N. Lange: 5-Aminolevulinic acid and its derivatives: physical chemical properties and protoporphyrin IX formation in cultured cells . In: J Photochem Photobiol B. , 2000 Jan, 54 (1), pp. 72-80, PMID 10739146 .
- ↑ JM Gaullier, K Berg, Q Peng, H Anholt, PK Selbo, LW Ma, J. Moan: Use of 5-aminolevulinic acid esters to improve photodynamic therapy on cells in culture . In: Cancer Res . , 1997 Apr 15, 57 (8), pp. 1481-1486, PMID 9108449 .
- ^ RG Tunstall, AA Barnett, J Schofield, J Griffiths, DI Vernon, SB Brown, DJ. Roberts: Porphyrin accumulation induced by 5-aminolaevulinic acid esters in tumor cells growing in vitro and in vivo . In: Br J Cancer. , 2002 Jul 15, 87 (2), pp. 246-250, PMID 12107850 .
- ↑ L Rodriguez, A Battle, G Di Venosa, AJ MacRobert, S Battah, H Daniel, A. Casas: Study of the mechanisms of uptake of 5-aminolevulinic acid derivatives by PEPT1 and PEPT2 transporters as a tool to improve photodynamic therapy of tumors . In: Int J Biochem Cell Biol. 2006, 38 (9), pp. 1530-1539, PMID 16632403 .
- ↑ JB Lee, JY Choi, JS Chun, SJ Yun, SC Lee, J Oh, HR. Park: Relationship of protoporphyrin IX synthesis to photodynamic effects by 5-aminolaevulinic acid and its esters on various cell lines derived from the skin . In: Br J Dermatol . , 2008 Jul, 159 (1), pp. 61-67, PMID 18489589 .
- ↑ a b c R Schulten, B Novak, B Schmitz, H. Lübbert: Comparison of the uptake of 5-aminolevulinic acid and its methyl ester in keratinocytes and skin . In: Naunyn Schmiedebergs Arch Pharmacol . , 2012 Oct, 385 (10), pp. 969-979, PMID 22801976 .
- ↑ Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomized controlled multicenter phase III trial . In: Lancet Oncol . 2006, 7, pp. 392-401.
- ↑ Technical information Gliolan 30 mg / ml as of 02/2014 www.fachinfo.de accessed October 9, 2017.
- ↑ LR Braathen, RM Szeimies, N Basset-Seguin, R Bissonnette, P Foley, D Pariser, R Roelandts, AM Wennberg, CA. Morton: Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: an international consensus . International Society for Photodynamic Therapy in Dermatology, 2005. In: J Am Acad Dermatol 2007, 56, pp. 125-143, PMID 17190630 .
- ↑ CA. Morton, RM Szeimies, A Sidoroff, LR. Braathen: European guidelines for topical photodynamic therapy part 1: treatment delivery and current indications - actinic keratoses, Bowen's disease, basal cell carcinoma . In: J Eur Acad Dermatol Venereol. , 2013 May, 27 (5), pp. 536-544, PMID 23181594 .
- ^ T Maisch, F Santarelli, S Schreml, P Babilas, RM. Szeimies: Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model . In: Experimental Dermatology , 2010, 19, pp. E302-e305, PMID 19845760 .
- ↑ a b T Dirschka, P Radny R, Dominicus, H Mensing, H Brüning, L Jenne, L Karl, M Sebastian, C Oster-Schmidt, W Klövekorn, U Reinhold, M Tanner, D Gröne, M Deichmann, M Simon, F Hübinger, G Hofbauer, G Krähn-Senftleben, F Borrosch, K Reich, C Berking, P Wolf, P Lehmann, M Moers-Carpi, H Hönigsmann, K Wernicke-Panten, S Hahn, G Pabst, D Voss, M Foguet , B Schmitz, H Lübbert RM, Szeimies: Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a multicentre, randomized, observer-blind phase III study in comparison with a registered methyl-5-aminolaevulinate cream and placebo . AK-CT002 Study Group, AK-CT003 Study Group. In: Br J Dermatol. , 2012 Apr, 168 (4), pp. 825-836, PMID 21910711 .
- ↑ RM Szeimies, P Radny, M Sebastian, F Borrosch, T Dirschka, G Krähn-Senftleben, K Reich, G Pabst, D Voss, M Foguet, R Gahlmann, H Lübbert, U. Reinhold: Photodynamic therapy with BF-200 ALA for the treatment of actinic keratosis: results of a prospective, randomized, double-blind, placebo-controlled phase III study . In: Br J Dermatol. , 2010 Aug, 163 (2), pp. 386-394, PMID 20518784 .
- Jump up ↑ A Hauschild, E Stockfleth, G Popp, F Borrosch, H Brüning, R Dominicus, H Mensing, U Reinhold, K Reich, AC Moor, M Stocker, C Ortland, M Brunnert, RM. Szeimies: Optimization of photodynamic therapy with a novel self-adhesive 5-aminolaevulinic acid patch: results of two randomized controlled phase III studies . In: Br J Dermatol. , 2009 May, 160 (5), pp. 1066-1074, PMID 19222455 .
- ↑ G Dragieva, BM Prince, J Hafner, R Dummer, G Castle, U Binswanger, W. Kempf: A randomized controlled clinical trial of topical photodynamic therapy with methyl aminolaevulinate in the treatment of actinic keratoses in transplant recipients . In: Br J Dermatol. , 2004 Jul, 151 (1), pp. 196-200, PMID 15270891 .
- ↑ AR Oseroff, S Shieh, NP Frawley, R Cheney, LE Blumenson, EK Pivnick, DA. Bellnier: Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy . In: Arch Dermatol. , 2005 Jan, 141 (1), pp. 60-67, PMID 15655143 .
- ↑ S Karrer, E Kohl, K Feise, D Hiepe-Wegener, S Lischner, W Philipp-Dormston, M Podda, W Prager, T Walker, RM. Szeimies: Photodynamic therapy for skin rejuvenation: review and summary of the literature - results of a consensus conference of an expert group for aesthetic photodynamic therapy . In: J Dtsch Dermatol Ges. , 2013 Feb, 11 (2), pp. 137-148, PMID 23190505 .
- ↑ T Dirschka, P Radny, R Dominicus, H Mensing, H Brüning, L Jenne, L Karl, M Sebastian, C Oster-Schmidt, W Klövekorn, U Reinhold, M Tanner, D Gröne, M Deichmann, M Simon, F Hübinger , G Hofbauer, G Krähn-Senftleben, F Borrosch, K Reich, C Berking, P Wolf, P Lehmann, M Moers-Carpi, H Hönigsmann, K Wernicke-Panten, S Hahn, G Pabst, D Voss, M Foguet, B Schmitz, H Lübbert RM, Szeimies: Long-term (6 and 12 months) follow-up of two prospective, randomized, controlled phase III trials of photodynamic therapy with BF-200 ALA and methyl aminolaevulinate for the treatment of actinic keratosis . AK-CT002 Study Group, AK-CT003 Study Group. In: Br J Dermatol. , 2013 Apr, 168 (4), pp. 825-836, PMID 23252768 .
- ↑ Jan Koolman, Klaus-Heinrich Röhm: Pocket Atlas of Biochemistry. 3. Edition. Georg Thieme Verlag, 2003, ISBN 978-3-13-759403-1 , p. 192.
- ↑ Rainer Braun: Special toxicology for chemists: a selection of toxic substances. Vieweg + Teubner Verlag, 1999, ISBN 978-3-519-03538-1 , p. 38.
- ^ Resolution CM / ResAP (2011) 1 on quality and safety assurance requirements for medicinal products prepared in pharmacies for the special needs of patients . Council of Ministers of the European Union, January 19, 2011.
- ↑ 5-aminolevulinic acid as fertilizer (Pentakeep) (PDF; 266 kB).