Lead poisoning
| Classification according to ICD-10 | |
|---|---|
| T56.0 | Toxic effect: lead and its compounds |
| ICD-10 online (WHO version 2019) | |
In the lead poisoning or saturnism is an acute or chronic poisoning by the inclusion of metallic lead or lead compounds . The heavy metal is harmful to many living things.
This article looks at lead poisoning from lead compounds in humans . Lead and lead compounds can be absorbed through food, inhalation or through the skin . With a single intake, only comparatively large amounts (lethal dose of the readily water-soluble lead salt lead (II) acetate for adults: 5–30 g) of lead or lead compounds lead to acute lead poisoning; on the other hand, a lead dose of around 1 mg per day via food leads to chronic poisoning after a long period of time because lead is only excreted slowly and therefore accumulates in the body (especially in the bones instead of calcium ). The World Health Organization (WHO) estimates the average daily oral lead intake to be around 100–500 µg per person. The use of lead and lead compounds, e.g. B. The anti-knocking agent tetraethyl lead in car fuels, which burns to form inorganic lead compounds in the engine, as an essential lead source has declined sharply since the 1970s. At the same time, the measurable pollution of the environment with lead was also reduced.
Lead damages the central and peripheral nervous system, impairs blood formation and leads to gastrointestinal complaints and kidney damage. With a few exceptions, lead compounds are classified as toxic to reproduction (teratogenic and impaired fertility). Since July 2006, the German Research Foundation has rated lead and its inorganic compounds as "carcinogenic in animal experiments". Severe poisoning leads to coma and death from circulatory failure .
Organic lead compounds such as B. formerly known as antiknock agents used tetraethyl lead and tetramethyl-lead are, besides, also acute highly toxic because they in addition to the toxic metal contained, in addition to aggressive radicals decompose.
Lead poisoning is recognized as an occupational disease under the BG code 1101 .
Sources and intake of lead, incidence of lead poisoning
Lead was one of the first metallic materials used by humans. In Roman times, metallic lead was used on a large scale for water pipes, vessels and other devices and objects. The sweet-tasting but poisonous lead compound lead (II) acetate ("lead sugar") was used to sweeten and beautify wine. The first reports of lead poisoning (Latin saturnism ) date from this time.
After the French naval doctor Amédée Lefèvre (1798–1869) had shown that lead poisoning can cause intestinal colic ( Colique sèche ) and can result from both professional handling of lead and from drinking from lead-containing vessels, the naval ministry implemented its suggestions for improvement, which became clear after 1862 fewer cases of saturnism or acute lead poisoning occurred.
An examination of 5000 year old human bones from Sudan showed a lead content of 600 µg / kg, while bones from autopsy material in Munich contained 6500–9000 µg / kg in 1980. From this it is concluded that mankind has caused most of today's lead pollution itself through large-scale use of this metal. In Germany, health regulations and a work ban for women and young people in lead paint and lead sugar factories were issued as early as 1886 by the Federal Council. Together with the use of lead, lead pollution has fallen sharply in recent decades: In 1971, the Rhine at the Dutch border still carried 2000 t of lead per year; in 1984 only 500 t per year. The lead input into the surface waters on German soil in the catchment area of the Baltic Sea decreased by 70% between 1985 and 2000.
Many painters died as a result of lead poisoning. Examples:
- The German painter August Haake developed symptoms of lead poisoning in the fall of 1914 after using white lead as a white pigment for seven years . He died on January 2, 1915, at the age of 25, during a stomach operation that had become necessary because of lead poisoning.
- The Brazilian painter Candido Portinari (1903–1962) suffered health damage from the lead in paint. He spent the last decade of his life in poor health and died in 1962 as a result of lead poisoning.
- Guy Rose (1867–1925, an American painter) had symptoms of lead poisoning during his studies, from which he suffered until his death. He therefore avoided oil painting at times. In 1921 he suffered a stroke; He died in 1925.
Above all, the abolition of leaded gasoline (around 1985–2000) has greatly reduced lead pollution in the environment; Improved exhaust gas purification with fine dust filters in large incineration plants, better waste water and exhaust air purification in lead-processing plants and, in general, the reduced use of lead-containing products in industry have also contributed to this. Aviation fuel containing lead is still available (for details see AvGas # usage ).
Adults take in more than 80% of lead through food. When young children swallow lead-contaminated soil and dust, they can ingest more lead than they do with food. The amount of lead absorbed per day and kilogram of body weight is between 0.5 and 30 µg. It is relatively higher in children with an average of 0.8 µg / kg than in adults with an average of 0.55 µg / kg. The WHO specifies a 'Provisional Tolerable Weekly Intake' (PTWI; roughly translated as 'temporary / pending tolerable weekly intake') of 25 µg / kg body weight. This corresponds to about 3.6 µg / kg per day or about 200 µg for an adult weighing 60 kg.
Worldwide incidence of lead poisoning
According to a 2020 study by Unicef , 800 million children around the world have at least 5 micrograms per deciliter of lead in their blood, which is an increased concentration that can have negative health effects. The study author also assumes more than 900,000 premature deaths per year in adults that can be traced back to lead poisoning.
Recycling of highly toxic heavy metals in developing and emerging countries
According to the Unicef study, minors in poor nations, in Central and South America and in Eastern Europe , but most of them in Africa and Asia , are exposed to highly toxic heavy metals . The biggest trigger for lead poisoning is the recycling of used lead-acid batteries that come from vehicles.
Air pollution
The lead-based anti - knock agent tetraethyl lead significantly increased lead pollution in the air between around 1920 and 1980. Combustion in the engine produces lead and lead (II) oxide , which reacts with chlorine and bromine from the halogenated hydrocarbons added to the gasoline to form fine lead (II) chloride and lead (II) bromide particles. These suspended solids then settled mainly in the vicinity of the streets, where, compared to places further away from the streets, they led to a lead content of two to ten times greater in fruit and vegetables growing above ground. When lead is smelted and also refined, it is released as fine lead (II) oxide dust and increases lead pollution in the environment. Also, waste incineration plants and other combustion plants, as well as lead-processing plants emit lead-containing dust. In June 2005 the Federal Environment Agency (UBA) announced:
“Other heavy metals such as lead and mercury are also held up in the filters of the waste incineration plants. ... Here, too, the decline is impressive: Whereas in 1990 57,900 kilograms (kg) of lead and 347 kg of mercury were emitted from the incineration of household waste in waste incineration plants, the values went to 130.5 kg (corresponding to 0.2% of the initial emissions) and 4 , 5 kg (1.3% ...) back. So ... [they] no longer play a role in human pollution. "
The fine dusts (particle size: 0.1–10 µm) are sometimes carried by the wind to regions further away, where they are mainly deposited with the precipitation. Dust that contains a lot of lead is also formed when old red lead coatings are removed during corrosion protection measures (by sanding or sandblasting ).
An increased lead concentration in the blood can also occasionally be found in sport shooters and those who regularly train with firearms in closed rooms. The reason for this is lead-containing compounds ( lead azide and lead syphat ) in the primer caps of the ammunition, which release fine lead dust when firing
Lead from inhaled dust does not accumulate in the lungs, but is either absorbed or swallowed with the bronchial mucus conveyed into the throat as part of the self-cleaning of the airways (see ciliated epithelium ). Depending on their size, 30–50% of the inhaled lead particles remain in the lungs; a total of about 30% of the inhaled amount of lead is absorbed. With 20 m³ of air inhaled per day and a lead content of 1 µg / m³ in the breath - this value is seldom exceeded even in cities - this results in lead absorption of 6 µg per day. Since January 1, 2005, an annual average of 0.5 µg / m³ has been in effect.
Workers in lead extraction or in the manufacture and processing of products containing lead, such as lead-acid batteries, are particularly at risk . Air concentrations between 80 and 4000 µg / m³ can occur in lead smelters and refineries. The maximum workplace concentration (MAK value) of lead is set at 100 µg / m³ (0.1 mg / m³).
The Standard Oil Company operated a facility for the production of the anti-knock agent tetraethyl lead in a refinery in Bayway (New Jersey) in the early 1920s. Soon the workers who were busy with the production began to behave strangely, so that the building was nicknamed The loony gas building among the workers ( Eng .: "The crazy gasoline building"). In the autumn of 1924 the condition of the workers deteriorated rapidly. 32 of the 49 workers were hospitalized, 5 of them died. The Office of Chief Medical Examiner of the City of New York (OCME), headed by Charles Norris , was appointed to conduct the investigation. Alexander O. Gettler was able to prove lead poisoning as the cause of death. After Norris submitted the investigation report, New York City , New Jersey and Philadelphia banned leaded gasoline additives , among others . This ban was lifted again by the federal government in 1926. Norris kept an eye on the problem, however, and in 1934 was able to show that the concentration of lead in road dust had increased by 50 percent since 1924.
Food and drinking water
The lead content of food fluctuates greatly. Certain organisms accumulate lead; some fungi can reach concentrations of up to 40,000 µg / kg dry weight or even 80,000 µg / kg. 0.5–1000 µg / kg were found in freshwater fish. With a few exceptions, higher plants absorb only a small amount of lead via the roots or the leaf surface; This is why plant-based foods are mainly contaminated by the lead-containing dust that has settled on the surface, some of which can be washed off. In 1996, maximum values of up to 20,000 µg / kg were measured in plants with large leaf areas. Compared with most foods of animal origin (lead content 10–100 µg / kg), the innards of animals contain a relatively high amount of lead with 100–1000 µg / kg.
The European Community has individually determined maximum lead levels for many food groups, for example between 20 µg / kg for milk and 1500 µg / kg for mussels. The values depend on the amount of the food in question and how much lead the food normally contains. This is the reason for the high limit value in mussels , which accumulate lead ( bioaccumulation ) and therefore naturally contain a comparatively high amount of lead, but are typically not consumed in large quantities on a regular basis.
In the past, lead dishes and dishes made from tin were important sources of lead because acidic foods such as wine or fruit juice in particular could dissolve significant amounts of lead from them. Their use is now prohibited. As of 2020, lead-containing ceramic glaze can still release lead in relevant quantities to food. The maximum value for lead released into foodstuffs set in 1984 is 4000 µg / l, which is now regarded as too high.
Water pipes made of lead are particularly problematic with soft or acidic water, because a protective layer of poorly soluble lead (II) carbonate cannot form and in extreme cases up to 3000 µg / l (other sources: 200 µg / l, 500 µg / l ) Lead can go into solution. This means that the limit value for drinking water of 25 µg / l that has existed since December 1, 2003 , which had fallen to 10 µg / l by the beginning of 2013, can be exceeded many times over. For a long time, a limit of 40 µg / l applied. Lead pipes have not been installed since 1973, but are still present in 10–15% of households in metropolitan areas. Lead can also migrate from brass fittings into drinking water and exceed the current limit value if it remains in contact with the metal for a long time.
Small children in particular can absorb lead from contaminated soil; the lead content in the area close to the surface fluctuates extremely depending on the lead input: it can be less than 50,000 µg / kg or greater than 1,000,000 µg / kg.
Adults only absorb about 10% of the ingested amount of lead into the body via the digestive tract, while in children between the ages of two months and six years up to 50% of the lead enters the body. Therefore, children are particularly at risk from lead in their diet.
Smoke
Heavy metal cations (cadmium - 0.007–0.35 micrograms per cigarette -, mercury, copper, arsenic, nickel, zinc, lead, antimony and gold) are found in tobacco smoke . This is another cause of health damage from smoking .
Since the summer of 2006, cannabis to which elemental lead had been added as an extender has repeatedly appeared in Germany . In the Leipzig area, 29 lead poisoning was documented that can be traced back to its consumption. In February 2009, several lead poisonings were found in the Munich area after people had consumed cannabis extended with lead sulfide . High levels of lead were found in the blood of patients suffering from symptoms of intoxication.
Other causes
An increased lead intake can rarely also be triggered by pathological behavior or special hobbies or habits, which are often very difficult to identify as the cause:
- The Pica syndrome is a rare eating disorder , take in the human things to be commonly referred to as inedible or even nauseous be considered. This can lead to the absorption of old lead-containing paint residues, lead-containing earth or lead-containing clay.
- Illegal alcohol burning is sometimes done using old heating radiators that have leaded soldered seams.
- Hobbies with glass blowing , glass dyeing , ceramic painting, in which leaded glasses and colors are used.
- Imported spices and dietary supplements , especially turmeric - or turmeric -Präparate be in some countries with lead chromate mixed, in order to improve their color and to increase their weight and thus the price.
- Alternative and complementary remedies, also from traditional Chinese medicine and Indian Ayurveda medicine, which can be lead-contaminated. Examples are Albayalde, Anzroot, Azarcon, Bali Goli, Ghasard, Greta, Jin Bu Huan, Koo Sar, Kushta, Litargirio.
- Imported cosmetics and religious powders such as Swad (from Hinduism), Tiro (eye cosmetics from Nigeria), Kohl or Surma (eye cosmetics from Africa, the Middle and Far East), hair dyes with lead acetate.
Lead in the body
distribution
A distinction is made between three lead depots in the body, which can store and release lead at different speeds and strive for a reciprocal equilibrium: First, ingested lead enters the blood , where 95% of it binds to the red blood cells . A balance is quickly established between blood and heart , lungs , liver , kidneys , brain and digestive tract. The exchange with muscles and skin is slower. In soft tissue, lead has a half-life of around 20 days. The lead in these deposits is either excreted or stored in the bones and teeth as lead phosphate instead of calcium phosphate . There it forms a very long-lasting depot with a half-life of 5–20 years. In adults, 90% of the lead in the body is in the bones, in children only 60%. When bone substance is broken down, e.g. B. in the case of calcium deficiency, stress, during cortisone therapy, due to acidosis or during pregnancy, the blood lead level can rise and trigger symptoms of acute lead poisoning, the so-called lead crisis, without additional lead from outside.
Lead crosses the placenta ; thus, lead from the blood of a pregnant mother can pass onto the unborn child and cause damage.
effect
| impact | Blood concentration in µg / l (children) | Blood concentration in µg / l (adults) |
|---|---|---|
| Slightly impaired kidney function | 100 | - |
| Statistically lower IQ | 100-200 | - |
| Statistically earlier due date | - | 140 |
| Enzyme inhibition in blood formation | from 150 | from 150 |
| BGW (women under 45 years of age) | - | 300 |
| Urinary delta aminolevulinic acid and coproporphyrin increased | 400 | 400 |
| BGW | - | 400 |
| Visuomotor performance decreased | 100-200 | 500 |
| Anemia | 200 | 500 |
| chronic encephalopathy | 500-600 | 800 |
| Paralysis | 600-800 | 600-800 |
| acute encephalopathy | 800-1000 | 1200 |
The effect of lead on the body depends on the concentration of lead in the blood. For adult men, a biological limit value of 400 µg / l is the highest permissible value in the workplace, for women under 45 years of age a value of 300 µg / l. Since animal experiments and large-scale studies in children and pregnant women have found evidence of lead in the blood from around 150 µg / l lead that IQ and learning behavior are impaired, according to the WHO a blood lead level of 100 µg / l should be in 98% of the population Children included) must not be exceeded.
Smooth muscles contract due to lead ( muscle contraction ), which leads to bowel cramps. In addition, small blood vessels narrow; this makes the skin appear pale and the blood pressure rises slightly.
Blood formation
Lead inhibits three enzymes involved in blood formation : delta aminolevulinic acid dehydratase (ALAD), coproporphyrinogen oxidase (outdated "coprogenase") and ferrochelatase . , This results in that on one hand the formation of blood is generally disturbed, and on the other hand, intermediates (the substrates of the enzymes in question or its metabolic products. E. Δ-aminolevulinic acid (ALA), the dehydrogenation product coproporphyrin III of Koproporphyrinogen III and protoporphyrin IX ) accumulate.
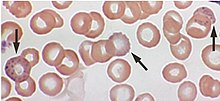
The inhibition of ALAD can be demonstrated from a blood lead level of around 150 µg / l, and from 400 µg / l the ALA content in the urine is increased. The brown coproporphyrin III can also be increasingly detected from this blood lead concentration in the urine and turns it dark brown in more severe cases (high blood lead level). It also contributes to the pale-gray-yellow coloration of the skin in chronic lead poisoning. The protoporphyrin content in the red blood cells rises between 200 and 600 µg / l and causes so-called "basophilic stippling" of the red blood cells, which can be detected microscopically.
Above 500 µg / l (200 µg / l in children) lead in the blood can cause anemia because it restricts blood formation and reduces the lifespan of red blood cells. In addition to anulocytes occur.
Cardiovascular system
Lead causes blood pressure to rise because vasodilating hormones ( prostaglandins ) are released less . Instead, vasoconstrictors are produced. Cardiac arrhythmias also occur due to disruption of the cellular calcium balance. Lead also increases the risk of heart attacks, heart failure and arrhythmias, mostly as a result of atherosclerosis . This is promoted, among other things, by the formation of free radicals and the increase in smooth muscle cells in the blood vessels.
Nervous system
In children from 100–200 µg / l lead in the blood, statistical psychological changes such as slightly reduced intelligence and psychomotor deficits can be determined. In adults, the performance in visimotor tests is reduced from 500 µg / l. To encephalopathy occurs in adults from 1200 ug / L in children starting at 800-1000 ug / l. This encephalopathy is often fatal in children if left untreated and often causes permanent neurological and neuropsychological damage in survivors. Symptoms of such encephalopathy include headache, disorientation, insomnia, vomiting, apathy , stupor , overactivity, and aggressiveness. In severe cases it leads to delirium , convulsions, coma and death from circulatory failure.
Chronic lead poisoning with blood concentrations from 600–800 µg / l paralyzes extensors that are more stressed due to nerve damage . The first sign of chronic lead poisoning is often the falling hand, in which the hand opposite the forearm cannot be raised. This paralysis, also known as the "falling hand position", is the result of damage to the radial nerve (radial nerve) .
Digestive organs
Lead poisoning causes typical intestinal colic (" lead colic ") and constipation, in severe cases a spastic ileus . Acute poisoning from large amounts of a lead compound can induce vomiting; the vomit may be colored white by the lead (II) chloride formed in the stomach with the gastric acid .
Kidneys
Kidney function is slightly impaired in children with 100 µg / l lead in the blood and more. In severe cases of poisoning, the kidney tissue can be damaged. In rats, lead in high doses over long periods of time has caused kidney tumors .
Reproduction
In animal experiments in rats and primates, blood lead levels of around 150 µg / l before birth led to permanent learning and memory deficits. In animal experiments, lead has also reduced the litter size, birth weight and survival rate of newborns. In humans, a mother's blood lead level of more than 140 µg / l seems to be able to reduce the age at birth ( premature birth ).
excretion
Lead is only excreted very slowly, so that normal values will only set in after years of lead intake. Most (76%) of the lead is eliminated through the kidneys; via bile and intestines 16% and less than 8% with hair, nails, peeling skin and sweat.
Diagnosis and monitoring
The external symptoms of lead poisoning include the so-called “lead color” (pale-gray-yellow discoloration of the skin), intestinal colic, diffuse central nervous symptoms such as fatigue, headache, loss of appetite, etc., paralysis, a blue-black containing lead (II) sulfide "Lead fringes" in the gums around the necks of the teeth and, in severe cases, encephalopathy .
Blood, urine, teeth, and hair can be used to determine human lead exposure. Blood is best for diagnosing and monitoring the success of a treatment.
The lead content of urine fluctuates too much depending on fluid consumption and kidney function; In addition, the lead content in urine is 10 times lower than that in blood and therefore more difficult to determine. Since the content of ALA and coproporphyrin III in the urine is only significantly increased from the already damaging blood lead concentration of 400 µg / l , these parameters are only suitable for increasing exposure - e.g. B. work-related - to recognize. This parameter is unsuitable for monitoring lead exposure in the population.
In principle, teeth are suitable for determining the long-term exposure to lead, because the lead content increases with age and lead exposure. However, they are generally not available to adults; however, they are well suited to determining lead exposure in children because their deciduous teeth can be examined.
The exposure of the previous months can be estimated from the deposits in the hair; however, the result can be falsified by lead adhering to the hair.
The blood lead level is less suitable for detecting lead deposits in the bones due to previous exposure, because it is often no longer elevated. Instead, you can either determine the lead in the bones directly by means of X-ray fluorescence analysis, or you can carry out an EDTA provocation test. To do this, the patient is injected with EDTA and the lead excretion is monitored. If it is significantly higher than a typical comparison value, this indicates that the lead depot in the body is too large.
treatment
After oral ingestion of a lead-containing substance, attempts are first made to prevent the lead from being absorbed by vomiting and gastric lavage. Acute poisoning is counteracted with gastric lavage with a three percent sodium sulfate solution and simultaneous administration of activated charcoal. As a result, lead ions are converted into poorly soluble lead sulfate and bound to the carbon.
In order to remove lead from the blood and from easily available deposits, therapy with chelating agents such as sodium DMPS (Dimaval) EDTA, DTPA and / or D-penicillamine can be considered. The chelates are excreted through the kidneys. However, chelating agents cause severe side effects and cannot be given over a longer period of time because they also flush essential trace elements from the body. Chelation therapy in children should be considered if the blood lead level is above 450 µg / kg.
After poisoning with an unclear cause, it is important to identify the lead source (s) and turn it off to prevent re-intoxication.
Prevention
The only protection against lead is to try not to release and ingest it. Many uses for lead are now banned or severely restricted; These include white lead as a white pigment, red lead as rust protection, tetraethyl lead as an anti - knock agent , lead-containing solder and many more. Although non-leaded alternatives are available for lead shot , lead shot has not yet been banned across the board. Where lead cannot be replaced, production processes should be used that produce as little lead-containing dust and lead-containing waste water as possible. If dust and wastewater are unavoidable, they must be sucked off or captured, collected and cleaned and then disposed of appropriately. In order to protect potentially endangered workers who could come into contact with lead or compounds containing lead, employers and employees must comply with the relevant occupational health and safety regulations. This includes monitoring the air concentration, monitoring the blood lead level among staff, hygiene measures and, if necessary, protective equipment.
Products containing lead and waste such as old car batteries may have to be collected and disposed of separately.
For people who do not come into contact with lead at work or who live in heavily contaminated areas, there is now usually no longer any risk of ingesting too much lead through air and food. The still existing lead water pipes are problematic as they can release considerable amounts of lead into drinking water. If possible, they should be replaced with water pipes made of suitable material. "Letting" the water that has been in the pipe run off for a long time is a sensible measure for adults, but not sufficient for babies and pregnant women. When preparing baby food, water with a high lead content must be avoided at all costs, as children are particularly at risk from lead in food. Fruits and vegetables should be washed off before consumption in order to remove adhering dust. Foods with a naturally high lead content such as wild mushrooms, mussels and offal should only be consumed in small quantities.
For food you may only use containers and devices that are expressly marked as suitable. Ceramic vessels coated with lead-containing glaze can be problematic; Caution is advised with "no-name products" and imports from abroad. In 2007, it was discovered that toys were coated with paint that was disproportionately high in lead. There were several product recalls, for example by the American company Mattel , which had cheap production in the People's Republic of China , with quality assurance not taking place or at least insufficient.
According to a study, iron and EDTA are said to lower the lead level in the blood somewhat. Iron enters the blood in the small intestine via the same transport protein as lead, so that more lead is absorbed in the event of iron deficiency.
See also
literature
- Paul Schmidt, Adolf Seiser , Stillfried Litzner: Lead poisoning . Urban & Schwarzenberg, Berlin a. a. 1930.
- Klaus Schümann: Historical aspects of lead poisoning . In: Bergknappe , 31, 2007, 1, pp. 14–22, complete issue (PDF; 5.2 MB)
- Franziska P. Busse, Georg Martin Fiedler, Alexander Leichtle, Helmut Hentschel, Michael Stumvoll: Lead Poisoning Due to Adulterated Marijuana in Leipzig . In: Deutsches Ärzteblatt . tape 105 , no. 44 , 2008, p. 757–756 , doi : 10.3238 / arztebl.2008.0757 , PMC 2696942 (free full text).
Web links
- Technical rules for hazardous substances: lead and hazardous substances containing lead (TRGS 505) (PDF; 66 kB)
- Bibliography for lead poisoning in ancient times (English)
- Information about lead-containing cannabis products from the German Hemp Association
- Leaflet on BK No. 1101: Diseases caused by lead or its compounds ( Memento from July 30, 2014 in the Internet Archive )
Individual evidence
- ↑ a b Reinhard Ludewig , Karlheinz Lohs : Acute poisoning. 6th edition. Gustav Fischer Verlag, Stuttgart 1981, ISBN 3-437-10697-X , pp. 127-129.
- ↑ a b c d e f C.-J. Estler (Ed.): Pharmacology and Toxicology . 5th edition. F. K. Schattauer Verlagsgesellschaft, Stuttgart 2000, ISBN 3-7945-1898-5 , pp. 735-738.
- ↑ a b c d e f Air quality guidelines for Europe . 2nd Edition. World Health Organization, Regional Office for Europe, Copenhagen 2000, ISBN 978-92-890-1358-1 , Chapter 6.7: Lead. ( who.int [PDF]).
- ↑ a b c d e f g h i j k l m n o p q r s Hans Marquardt, Siegfried G. Schäfer (Ed.): Textbook of Toxicology . Spektrum Akademischer Verlag, Heidelberg 1997, ISBN 3-8274-0271-9 , pp. 513-517.
- ↑ a b c Konrad Lang: Biochemistry of nutrition . 4th edition. Steinkopff, Darmstadt 1979, ISBN 3-7985-0553-5 , pp. 379-381.
- ↑ Guide to the use of environmentally friendly substances . (PDF; 982 kB) Federal Environment Agency, February 2003.
- ↑ German Research Foundation: DFG presents 2006 MAK and BAT value list ( Memento of February 10, 2009 in the Internet Archive ) (press release No. 34, July 5, 2006).
- ↑ Entry on tetraethyl lead. In: Römpp Online . Georg Thieme Verlag, accessed on April 10, 2017.
- ↑ Barbara I. Tshisuaka: Lefèvre, Amédée. In: Werner E. Gerabek , Bernhard D. Haage, Gundolf Keil , Wolfgang Wegner (eds.): Enzyklopädie Medizingeschichte. De Gruyter, Berlin / New York 2005, ISBN 3-11-015714-4 , p. 834.
- ↑ Collection of sources on the history of German social policy from 1867 to 1914 , Section II: From the Imperial Social Message to the February Decrees of Wilhelm II (1881–1890), Volume 3: Workers' Protection , edited by Wolfgang Ayaß , Darmstadt 1998, ISBN 3-534 -13440-0 , No. 26 and No. 121.
- ↑ Emissions from the Baltic catchment area. ( Memento from August 24, 2011 in the Internet Archive ) Federal Environment Agency, as of November 16, 2005.
- ^ Volkert HU Koch: August Haake 1889–1915. Published by the Kunstverein Fischerhude in Buthmanns Hof e. V. Verlag Atelier im Bauernhaus, Fischerhude 2006. Page 126.
- ↑ a b Heavy Metals in Food , Bavarian Consumer Information System (as of January 13, 2006).
- ↑ a b The toxic truth. Accessed August 18, 2020 .
- ↑ a b c d e Anne Backhaus, DER SPIEGEL: Invisible Danger: Lead in batteries, dishes and spices poisons millions of children - DER SPIEGEL - Politics. Retrieved August 18, 2020 .
- ↑ a b lead. ( Memento from December 31, 2006 in the Internet Archive ) Federal Environment Agency, status: November 18, 1998.
- ↑ Waste incineration - a source of danger? Farewell to the dioxin spinner . ( Memento of March 4, 2012 in the Internet Archive ) (PDF; 59 kB) p. 4 f. For the situation in 1998 see also the previous footnote.
- ↑ a b c d e Bruno Streit: Lexicon of Ecotoxicology . VCH, Weinheim 1991. ISBN 3-527-28104-5 , pp. 104-110.
- ↑ Air pollutant lead . ( Memento from October 7, 2006 in the Internet Archive ) (PDF) Federal Environment Agency, as of November 2005.
- ^ The Poisoner's Handbook (copy of a documentary broadcast on PBS ).
- ↑ Deborah Blum: Looney Gas and Lead Poisoning: A Short, Sad History .
- ↑ a b c d e f g h i j k l m n Substance monograph lead . (PDF) Commission “Human Biomonitoring” of the Federal Environment Agency. In: Bundesgesundheitsblatt , 1996, Volume 39, pp. 236–241.
- ↑ a b Lead and cadmium from ceramics . (PDF; 147 kB) Federal Institute for Risk Assessment, June 7, 2005.
- ↑ a b c Limit value for lead in drinking water lowered , Federal Environment Agency (December 12, 2003).
- ↑ Franziska Busse, Leyla Omidi, Alexander Leichtle, Michael Windgassen, Eyleen Kluge, Michael Stumvoll: Lead Poisoning Due to Adulterated Marijuana . In: New England Journal of Medicine . tape 358 , no. April 15 , 2008, p. 1641–1642 , doi : 10.1056 / NEJMc0707784 , PMID 18403778 (free full text).
- ↑ Lead-contaminated marijuana found in Bavaria .
- ↑ Rose H. Goldman, Lisa Weissmann: A Diagnosis to Chew On New England Journal of Medicine 2019, Volume 381, Issue 5, August 1, 2019, pages 466-473, DOI: 10.1056 / NEJMcps1900774
- ↑ TRGS 903 (PDF) Biological limit values, BAuA.
- ↑ Rainer Braun: Special toxicology for chemists: a selection of toxic substances. Vieweg + Teubner Verlag, 1999, ISBN 978-3-519-03538-1 , p. 38.
- ↑ Mercury and lead make it difficult for your heart . (PDF).
- ↑ Ernst Mutschler: drug effects, textbook of pharmacology and toxicology. 8th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 2001, ISBN 978-3-8047-1763-3 , p. 970 f.
- ↑ Drink something - drinking water from the tap (PDF; 468 kB) Federal Environment Agency.
- Jump up ↑ Lead in Fisher Price Toys. In: Focus Online . August 2, 2007. Retrieved June 9, 2017 .
- ↑ Iron works against toxic lead. In: Neue Zürcher Zeitung . October 25, 2016. Retrieved June 9, 2017 .