Ferrochelatase
| Ferrochelatase | ||
|---|---|---|
|
Existing structural data: s. UniProt |
||
| Properties of human protein | ||
| Mass / length primary structure | 369 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Cofactor | (2Fe-2S) | |
| Identifier | ||
| Gene name | FECH | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 4.99.1.1 , lyase | |
| Response type | elimination | |
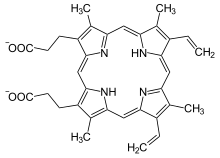
| Substrate | Protoporphyrin IX + Fe 2+ | |
| Products | Heme b + 2 H + | |
| Occurrence | ||
| Parent taxon | Creature | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2235 | 14151 |
| Ensemble | ENSG00000066926 | ENSMUSG00000024588 |
| UniProt | P22830 | Q544X6 |
| Refseq (mRNA) | NM_001012515 | NM_007998 |
| Refseq (protein) | NP_001012533 | NP_032024 |
| Gene locus | Chr 18: 57.54 - 57.59 Mb | Chr 18: 6.45 - 64.49 Mb |
| PubMed search | 2235 |
14151
|
Ferrochelatase (also: heme synthase) is an enzyme in eukaryotes and most bacteria , the last step of the synthesis of heme , the chelation of protoporphyrin IX with an iron -II-ion catalyzed . Since the reaction in eukaryotes takes place in the mitochondria or chloroplasts , protoporphyrin must first be transported into these compartments; the exact course of the transport is still unclear. In humans cause mutations on FECH - gene to Ferrochelatasemangel which for the rare hereditary disease erythropoietic Protoporphyrie is responsible. The iron-chelating enzyme has not yet been identified in archaea and sulfate-reducing bacteria , which produce heme using an alternative synthetic route.
Catalyzed reaction
Protoporphyrin IX is chelated with iron II, protons are split off, and heme b is formed. Conversely, the enzyme can also catalyze the removal of the metal ion during the breakdown of various hems. An iron-sulfur cluster acts as a cofactor . Recombinant ferrochelatase is also able to chelate other divalent metal ions such as cobalt , nickel , zinc or copper .
Individual evidence
- ↑ a b BRENDA entry
- ↑ Jassal, D'Eustachio / reactome: Protoporphyrin IX is transported from the mitochondrial intermembrane space into the mitochondrial matrix ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice. .
- ↑ UniProt P22830
- ↑ M. Kühner et al. : The Alternative Route to Heme in the Methanogenic Archaeon Methanosarcina barkeri . In: Archaea . 2014, 2014. doi : 10.1155 / 2014/327637 .
- ↑ S. Taketani, M. Ishigaki, A. Mizutani, et al. : Heme synthase (ferrochelatase) catalyzes the removal of iron from heme and demetalation of metalloporphyrins . In: Biochemistry . 46, No. 51, December 2007, pp. 15054-61. doi : 10.1021 / bi701460x . PMID 18044970 .
- ↑ Hunter GA, Sampson MP, Ferreira GC: Metal ion substrate inhibition of ferrochelatase . In: J. Biol. Chem. . 283, No. 35, August 2008, pp. 23685-91. doi : 10.1074 / jbc.M803372200 . PMID 18593702 .
Web links
- Jassal, D'Eustachio / reactome: Ferrous iron is inserted into protoporphyrin IX to form heme