Porphobilinogen
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Porphobilinogen | |||||||||||||||||||||
| other names |
3- [5- (aminomethyl) -4- (carboxymethyl) -1 H -pyrrole-3-yl] propionic acid |
|||||||||||||||||||||
| Molecular formula | C 10 H 14 N 2 O 4 | |||||||||||||||||||||
| Brief description |
pink crystals (monohydrate) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 226.23 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
172-175 ° C |
|||||||||||||||||||||
| solubility |
bad in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
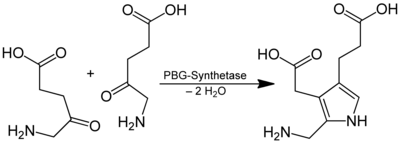
Porphobilinogen (PBG) is a precursor of heme in the porphyrin synthesis and is an important building block for biosynthetic build-up of porphyrins , Hydroporphyrinen and corrins . It is formed from two molecules of 5-aminolevulinic acid ( ALA ) with elimination of water, the catalyzing enzyme is here the δ-aminolevulinic acid dehydratase :
In the next step, four molecules of PBG are polymerized to hydroxymethylbilane using porphobilinogen deaminase .
Porphobilinogen can be detected in the urine in the case of porphyria . Lead inhibits δ-aminolevulinic acid dehydratase, which is why an elevated level of ALA in the urine and blood indicates lead poisoning .
Individual evidence
- ↑ a b c d e entry on porphobilinogen. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ a b data sheet Porphobilinogen at Sigma-Aldrich , accessed on April 21, 2011 ( PDF ).
- ^ The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals , 14th Edition (Merck & Co., Inc.), Whitehouse Station, NJ, USA, 2006; P. 1313, ISBN 978-0-911910-00-1 .
- ↑ Rainer Braun: Special toxicology for chemists: a selection of toxic substances. Vieweg + Teubner Verlag, 1999, ISBN 978-3-519-03538-1 , p. 38.
- ↑ Jan Koolman , Klaus-Heinrich Röhm: Pocket Atlas of Biochemistry. 3rd edition, Georg Thieme Verlag, 2003, ISBN 978-3-13-759403-1 , p. 192.