Strecker synthesis
The Strecker (amino acid) synthesis is a name reaction in organic chemistry . Its discoverer, Adolph Strecker (1822–1871), published it for the first time in 1850.
It is a special case of the Mannich reaction , in which α- amino acids or amino acids are formed from aldehydes , ammonia and hydrogen cyanide (HCN) . The synthesis is also valid for primary and secondary amines .
mechanism
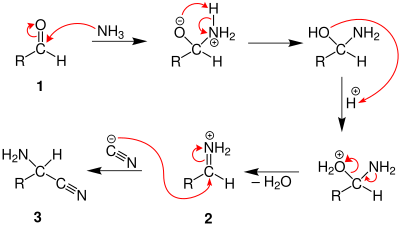
The reaction takes place via the nucleophilic addition of ammonia to aldehyde 1 . This generally runs up to Imin 2 . The cyanide adds to this also electrophilic species, creating an α - aminonitrile 3 (a nitrile with an amino group ):
The α - amino nitrile 3 is hydrolyzed several times in an acidic medium , which ultimately results in the α- amino acid 4 as a racemate :
practice
The main disadvantage of the Strecker synthesis is that, due to the lack of asymmetric induction, the amino acids are formed racemically . This increases the costs considerably because of the 50% reduction in yield per se and the subsequent complex separation processes, usually the kinetic resolution . Dealing with cyanide is also problematic due to its toxicity. Nevertheless, some Strecker syntheses have established themselves in large-scale industrial production, since the starting materials (especially ammonia and hydrogen cyanide ) are sometimes very inexpensive and there are still old plants designed for this synthesis route. The Bucherer-Bergs reaction , for example, is a variant used.
Asymmetric Strecker syntheses also exist. In the variant by Kunz et al. a chiral 1- amino sugar ( β - D - galactosamine ) is used instead of ammonia, the asymmetric induction of which is sufficient to achieve satisfactory enantiomeric excesses.
Another interesting approach to the asymmetric variant of the Strecker synthesis was developed by Enders' group. They use SAMP hydrazones as an asymmetric induction for the addition of the cyanide.
See also
Individual evidence
- ↑ Adolph Strecker: About the artificial formation of lactic acid and a new body that is homologous to Glycocoll. In: Annals of Chemistry and Pharmacy. 75, 1850, pp. 27-45, doi : 10.1002 / jlac.18500750103 .
- ^ László Kürti , Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis . Elsevier Academic Press, Burlington / San Diego / London 2005, pp. 446-447, ISBN 978-0-12-429785-2 .
- ↑ Jie Jack Li: Name reactions, a collection of detailed reaction mechanism . 5th edition. Springer 2014. pp. 591-592. doi : 10.1007 / 978-3-319-03979-4_268
- ↑ J. Mulzer , H.-J. Altenbach, M.Braun, K. Krohn, H.-U. Reissig, Organic Synthesis Highlights , VCH, Weinheim, 1991 , p. 303.
- ↑ H. Kunz et al. , Angew. Chem. 1987 , 99 , 595.
- ↑ D. Enders, M. Moser, Tetrahedron Lett. 2003 , 44 , 8479.