Resolution
As resolution method for separation of racemates into their enantiomers designated. Enantiomers of active ingredients in pharmaceuticals or crop protection agents usually have different biological activities. The resolution is used, among other things, to obtain these enantiomers in as pure a form as possible. Sales of enantiomerically pure drugs in 2000 were approximately $ 150 billion.
Until the development of asymmetric synthetic methods , racemate resolution was the only way to obtain pure enantiomers from racemic products, which are usually formed in chemical syntheses. The resolution of racemates is still a method that is frequently used in industry to produce enantiomerically pure substances.
history
Louis Pasteur succeeded in resolving racemates for the first time in 1848 by manually sorting out enantiomeric sodium ammonium tartrate crystals , which behave macroscopically like image and mirror image, under the microscope . This method is only successful if the racemate forms crystals through spontaneous cleavage which contain only one of the enantiomers. In 1857 Pasteur achieved the resolution in a different way: by separating diastereomers. The reaction of a racemic acid (or base ) with an optically active base (or acid) gave diastereomeric salts, the isomers of which could be separated into the enantiomers by fractional crystallization . Pasteur found a third method of racemate resolution: as early as 1858 he first used fermentation using the mold Penicillium glaucum , which he grew on racemic tartaric acid as a nutrient. While one enantiomer was being metabolized by the fungus, the other enantiomer remained in solution.
Marckwald and A. McKenzie reported in 1899 about the first kinetic resolution in the esterification of racemic mandelic acid with optically active (-) - menthol . In this reaction, the ( R ) -enantiomer of mandelic acid shows a higher reaction rate and ( S ) -mandelic acid is enriched in the reaction mixture .
The first gas chromatographic separation of D , L -amino acid racemic mixtures into their enantiomers using a chiral stationary phase was achieved in 1966 by Emanuel Gil-Av at the Weizmann Institute for Sciences in Rehovot , Israel .
In 1975, Merck applied for a patent for the production of methyldopa via racemate resolution using conglomerate crystallization.
In 2002 Degussa started the production of enantiomerically pure amino acids using the hydantoinase process, which is based on dynamic kinetic resolution.
Mechanical separation processes
The mechanical separation method used by Pasteur is only suitable for systems in which the enantiomers crystallize as enantiomerically pure conglomerates . In addition, only relatively small quantities can be separated in this way.
The separation of racemates is more successful by inoculating supersaturated racemate solutions with a small amount of a pure enantiomer of the same racemate and subsequent fractional crystallization. The separation of the enantiomerically pure crystal and the solution is done by mechanical processes such as filtration or centrifugation . Although this method has been used on an industrial scale, its scope is limited, since many racemates crystallize racemically and not as enantiomerically pure conglomerates.
Separation process via diastereomer formation
The enantiomers contained in a racemate can be converted into diastereomers, which have different physical properties, by reaction with a chiral reagent or contact with a chiral phase. Due to the differences in the physical properties, the resulting diastereomers can be separated using conventional methods such as chromatography, crystallization or distillation. The formation of the diastereomer does not necessarily have to take place via covalent formation; it can be diastereomeric transition states that arise, for example, through hydrogen bonds , such as in chiral chromatography. A prerequisite for this separation method is that the separation takes place faster than racemization or that stable isomers are present. Another prerequisite is that enantiomerically pure compounds are available from the chiral pool , for example , which can be used to form the diastereomers.
Chiral Chromatography
Racemates can be separated using all known chromatography methods such as thin layer chromatography, HPLC , column chromatography or gas chromatography. The latter is preferably used in the analysis of non-racemic mixtures of enantiomers to determine the enantiomeric excess.
The chiral phase can be stationary or, in the case of liquid chromatography, the eluent. The usual method in the organic chemical laboratory is to bring them into contact with chiral materials. For this purpose, chromatography selects either the mobile phase (eluent) or the optically active stationary phase . This leads to different retention of two enantiomers. A thin-layer chromatographic separation of enantiomers using an enantioselective stationary phase is known.
Larger amounts of sample can be separated using column chromatography. To produce the chiral stationary phase, for. B. an enantiomerically pure chiral auxiliary such as (+) - tartaric acid on a support, such as. B. silica gel fixed. The racemic solution is chromatographed with a conventional eluent. The individual enantiomers interact differently with the chiral matrix and leave the column at different retention times.
The difference in the standard free energy of formation of the diastereomeric transition states
- Δ S, R ΔG ≠ = RT ln α
(α: separation factor)
causes a difference in retention time.
A number of substances are used as chiral phases. The type of chiral recognition is different depending on the type of carrier material. Peptide phases mostly interact via hydrogen bonds and dipole-dipole interactions, while chiral metal complexes interact via complexation. Gas chromatography using cyclodextrin derivatives has been studied intensively .
Crystallization and distillation
The resolution of racemates by means of fractional crystallization is a widely used method. Here, diastereomeric salts are separated by adding an enantiomerically pure auxiliary and subsequent separation by fractional crystallization using their different physical properties. The use of tartaric acid or quinine as a component of the chiral pool is widespread.
Diastereomers which cannot be crystallized or are difficult to crystallize can be separated by distillation. The process is suitable, for example, for racemic acids or alcohol mixtures that can be converted into the esters.
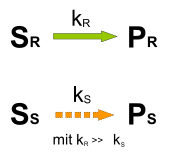
Kinetic resolution
If the rate constants for the conversion of the substrate isomers S R and S S into the corresponding products P R and P S are different, kinetic resolution is possible. The reaction is terminated at a conversion of about 50% because the faster reacting enantiomer has been consumed. The slower reacting enantiomer accumulates in the reaction mixture. The components S S and P R can be separated using conventional methods, P R can optionally be converted back into S R after separation has taken place .
In the kinetic resolution of racemate according to Willy Marckwald and McKenzie, racemic mandelic acid is partially esterified with optically active (-) - menthol . The ( R ) -enantiomer of mandelic acid shows a higher reaction rate and the ( S ) -mandelic acid accumulates in the reaction mixture . The enriched fraction can be separated off and the ( R ) -mandelic acid can be recovered by hydrolysis .
In contrast to fermentative resolution, kinetic resolution using enzymes has found wide application.
Dynamic kinetic resolution
The disadvantages of the kinetic resolution, such as the theoretically limited yield of 50% and the necessary work-up of the reaction solution, can be avoided by the dynamic kinetic resolution. By racemizing the slower reacting enantiomer S S , racemates can be converted quantitatively into products with a high enantiomeric excess. The yield and the enantiomeric excess can theoretically be 100%.
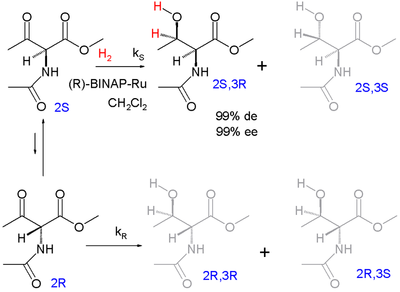
One of the first examples of dynamic kinetic resolution is asymmetric hydrogenation according to Ryoji Noyori (1989):
The enantiomers racemize via the enol form. The target product is the protected syn adduct L- threonine (2 S , 3 R ) with 99% diastereomeric excess (with preference for the syn -diastereomeric pair and not the anti-pair) and 99% enantiomeric excess (preference for the (3 R ) - Product within the syn pair).
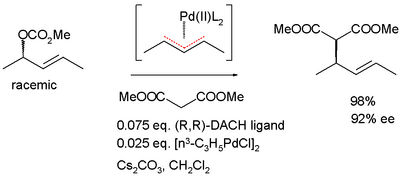
The dynamic kinetic resolution can take place via the formation of a prochiral transition state or a meso compound . An example of this is the allylic asymmetric alkylation according to Barry Trost , which takes place via an η 3 - palladium -allyl complex.
An example of the industrial application of dynamic kinetic racemate resolution is the hydantoinase process developed by Degussa for the preparation of amino acids with high enantiomeric purity. Hydantoins , which are quickly racemized by adding racemase , are used as substrates . By D -hydantoinase the (is R ) hydantoin to the carbamoyl-protected D cleaved amino acid, by a carbamoylase is then deprotected.
literature
- G. Subramanian: Chiral Separation Techniques: A Practical Approach , 641 pages, Verlag Wiley-VCH Verlag GmbH & Co. KGaA (2006) ISBN 3-527-31509-8 , ISBN 978-3-527-31509-3 .
- WA König: The Practice of Enantiomer Separation by Capillary Gas Chromatography , 104 pages, Verlag Hüthig (1987); ISBN 3-7785-1324-9 , ISBN 978-3-7785-1324-8 .
- PJ Walsh, MC Kozlowski: Fundamentals of Asymmetric Catalysis , 688 pages, Verlag Palgrave Macmillan (2008), ISBN 1-891389-54-8 , ISBN 978-1-891389-54-2 .
- A. Pandey: Enzyme Technology , 742 pages, Verlag Springer, Berlin (2006), ISBN 0-387-29294-2 , ISBN 978-0-387-29294-6 .
Web links
Individual evidence
- ^ V. Schurig: Importance of chirality and enantiomer separation - methods of chirality recognition, at uni-tuebingen.de. Retrieved September 21, 2013 .
- ^ Louis Pasteur: On the Relationships between the Crystalline Form, Chemical Composition and the Direction of Optical Rotation. Annales de Chimie et de Physique. Vol. 24. No. 6. 1848. pp. 442-459.
- ↑ Louis Pasteur: CR Hebd. Seance Acad. Sci. , 1857, vol. 45, p. 1032.
- ↑ Louis Pasteur: CR Hebd. Seances Acad. Sci. , 1858, Vol. 46, pp. 615-618.
- ↑ Willy Marckwald, A. McKenzie: About a principally new method for splitting racemic compounds into the active components. In: Ber. German Chem. Ges. 1899, 32, p. 2130. doi : 10.1002 / cber.189903202130
- ↑ Emanuel Gil-Av, Binyamin Feibush, Rosita Charles-Sigler: Separation of enantiomers by gas liquid chromatography with an optically active stationary phase. In: Tetrahedron letters 7.10 (1966): pp. 1009-1015.
- ↑ Edward JJ Grabowski: Enantiopure drug synthesis: From methyldopa to imipenem to efavirenz. In: Chirality. 17, 2005, pp. S249-S259, doi : 10.1002 / chir.20143 .
- ↑ Oliver May, Stefan Verseck, Andreas Bommarius, Karlheinz Drauz: Development of Dynamic Kinetic Resolution Processes for Biocatalytic Production of Natural and Nonnatural-Amino Acids. In: Organic Process Research & Development . 6, 2002, pp. 452-457, doi : 10.1021 / op020009g .
- ↑ Axel Kleemann and Jürgen Martens : Optical resolution of racemic S- (carboxymethyl) cysteine. In: Liebig's annals of chemistry. 1982, 11, pp. 1995-1998, doi : 10.1002 / jlac.198219821108 .
- ^ Kurt Günther, Jürgen Martens, Maren Schickedanz: Thin-layer chromatographic separation of enantiomers by means of ligand exchange. In: Angewandte Chemie. 96, 1984, pp. 514-515, doi : 10.1002 / anie.19840960724 .
- ↑ Volker Schurig , Hans-Peter Nowotny: Gas chromatographic separation of enantiomers on cyclodextrin derivatives. In: Angewandte Chemie. 102, 1990, pp. 969-986, doi : 10.1002 / anie. 19901020904 .
- ↑ Bernd Schäfer: Natural substances of the chemical industry , Elsevier GmbH, Spektrum Verlag, 2007, ISBN 978-3-8274-1614-8 , p. 155.
- ↑ Michal Shapira-Levinger, Ayelet Fishman: Kinetic resolution of a diltiazem intermediate by lipase-catalyzed enantioselective alcoholysis. In: Journal of Molecular Catalysis B: Enzymatic. 9, 2000, pp. 251-257, doi : 10.1016 / S1381-1177 (99) 00102-2 .
- ↑ R. Noyori, T. Ikeda, T. Ohkuma, M. Widhalm, M. Kitamura, H. Takaya, S. Akutagawa, N. Sayo, T. Saito: Stereoselective hydrogenation via dynamic kinetic resolution In: J. Am. Chem. Soc. 1989; 111, pp. 9134-9135; doi : 10.1021 / ja00207a038 .
- ↑ Barry M. Trost, Michelle R. Machacek, Aaron Aponick: Predicting the Stereochemistry of Diphenylphosphino Benzoic Acid (DPPBA) -Based Palladium-Catalyzed Asymmetric Allylic Alkylation Reactions: A Working Model. In: Accounts of Chemical Research. 39, 2006, pp. 747-760, doi : 10.1021 / ar040063c .