Cyclodextrins
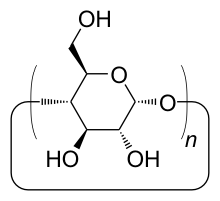
Cyclodextrins (CD) are a class of compounds that belong to the cyclic oligosaccharides . They represent ring-shaped degradation products of starch. They consist of α-1,4-glycosidically linked glucose molecules . This creates a toroidal structure with a central cavity. Cyclodextrins were first described by Villiers and Schardinger , but remained a laboratory curiosity for a long time. They were first isolated by Villiers in 1891 and characterized by Schardinger as oligosaccharides in 1903.
nomenclature
The cyclodextrins are named differently depending on the number of glucose units that make up them. Using a Greek letter as a prefix one differentiates:
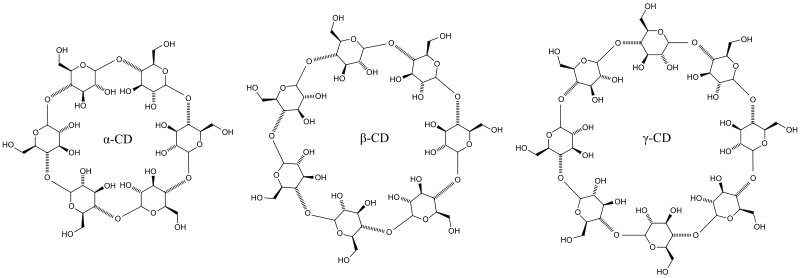
- α-cyclodextrin: n = 6 glucose molecules (cavity diameter / height: 4.7..5.3 / 7.9 Å )
- β-cyclodextrin: n = 7 glucose molecules (cavity diameter / height: 6.0..6.5 / 7.9 Å)
- γ-cyclodextrin: n = 8 glucose molecules (cavity diameter / height: 7.5..8.3 / 7.9 Å)
- δ-cyclodextrin: n = 9 glucose molecules
In addition to the above-mentioned cyclodextrins, cyclodextrins with significantly more glucose units are described in detail in the specialist literature. However, due to the small quantities and high prices, these are not of economic importance. Β-cyclodextrin and hydroxypropyl-β-cyclodextrin have the greatest technical distribution. In the food industry , α-cyclodextrin and γ-cyclodextrin are used. Since α-cyclodextrin is a soluble fiber , it can also be listed as such in the table of contents, e.g. B. as alpha-cyclodextrin (soluble fiber).
Extraction and manufacture
Cyclodextrins are produced biotechnologically through the enzymatic breakdown of starch, for example from corn or potatoes. The enzymes used for this are called cyclodextrin glycosyltransferases, or CGTases for short . When acting on starch, the CGTase cuts individual pieces out of the helically wound structure of the carbohydrate and connects them to form a ring-shaped oligosaccharide - the cyclodextrin. Of particular industrial interest is the single-variety extraction of cyclodextrins in order to be able to select the various cavity diameters depending on the substance to be enclosed. Selective enzymes are available, each specifically producing α-, β- and γ-cyclodextrin. This is particularly important for the food industry, since only α- and γ-cyclodextrin should be consumed indefinitely.
properties
All lower cyclodextrins have a hydrophobic cavity inside and a polar outer surface. As a result, the cyclodextrins are able to form so-called inclusion compounds with non-polar organic compounds.
Cyclodextrins are very stable in alkaline and acidic solutions up to pH 2 . They have no fixed melting points, but are stable up to around 200 ° C. They then begin to decompose. They are considered non-toxic and are largely stable to human digestive enzymes , which is why α-cyclodextrin can also be used as soluble fiber.
use
The ability to form inclusion compounds with apolar organic compounds and the water solubility make cyclodextrins an increasingly important subject of pharmaceutical research, since the complexes with pharmaceuticals are usually more water-soluble than the pure pharmaceuticals and are therefore also more readily available in the body. Furthermore, their ability to protect the enclosed substance from surrounding compounds (for example oxygen) and to release the enclosed substances over a longer period of time is of great interest.
α-Cyclodextrin has been approved as a soluble dietary fiber in the European Union since 2008. In June 2013 the Commission of the European Union certified α-cyclodextrin as having a proven health-promoting effect ( health claim ). The EU report confirms that α-cyclodextrin can reduce the rise in blood sugar after starchy meals. Due to its surface-active properties, it is used as an emulsifying fiber in the food ( mayonnaise ) and cosmetics industries ( skin creams ) and as a whipping agent for desserts , confectionery and baked goods . β-Cyclodextrin is approved in the EU as a food additive with the number E 459 with different maximum quantity restrictions for certain foods, in particular for the flavoring of teas or instant beverage powders (with 0.5 milligrams per kilogram in the intended consumption preparation) and snack products (with 1 Milligrams per kilogram) as well as for tableted foods (as required - quantum satis ). Gamma-cyclodextrin has been approved by the EU for Wacker Chemie AG as a novel food ingredient for food and beverage applications.
In addition, consumers come across cyclodextrins under the trade names Febreze , Bounce or Oust . The cyclodextrin derivatives in these products bind the compounds responsible for unpleasant odors and are also carriers of fragrances. In some countries (e.g. USA, Japan) cyclodextrins are permitted as food additives, as they can, among other things, cancel or release the taste and smell of the trapped substances. The range of uses of cyclodextrins today includes various areas of application, from various drug preparations and cardboard boxes to medicine, agriculture and sensor technology . The German Textile Research Institute in Krefeld is intensively researching the use of cyclodextrins covalently bound to the fabric fibers for "intelligent" textiles . These can contain drugs that can be absorbed through the skin and also bind sweat odor.
In analytical chemistry, and with particular success in gas chromatography , special cyclodextrin derivatives are used to separate mixtures of enantiomers . Cyclodextrin derivatives have also been successfully established in capillary electrophoresis ( CE ). With sulfated, charged cyclodextrins, it is even possible to separate neutral enantiomers that would otherwise not be accessible using CE.
Modified cyclodextrins were found to be virucidal according to a publication in 2020 ; further development of drugs or disinfectants is conceivable.
literature
- A. Villiers: Sur la transformation de la fécule en drextrine par le ferment butyrique. In: Compt. Rend. Ms. Acad. Sci. 1891, pp. 435-438.
- A. Biwer, G. Antranikian, E. Heinzle: Enzymatic production of cyclodextrins . In: Appl Microbiol Biotechnol. 59, 2002, pp. 609-617. PMID 12226716 .
Individual evidence
- ↑ Authorization to place α-cyclodextrin on the market as a novel food ingredient (2008/413 / EC) .
- ↑ Regulation (EU) No. 536/2013 of the Commission of June 11, 2013 amending Regulation (EU) No. 432/2012 to establish a list of permissible health-related claims about food other than information about the reduction of a disease risk and the development and Children's health (Text with EEA relevance) (Annex, 10 (6), 2012, pp. 2713 | 2926 |).
- ↑ Restricted additives , Annex 4 (to § 5 Paragraph 1 and § 7) of the Additive Approval Ordinance of January 29, 1998 (Federal Law Gazette I, p. 230, 231), last amended by Article 1 of the Ordinance of March 28, 2011 ( Federal Law Gazette I, p. 530) has been changed.
- ↑ WACKER receives approval for gamma-cyclodextrin as a new food ingredient for food and beverages in the EU . Press release Wacker Chemie AG, June 28, 2012.
- ↑ C. Bicchi: Cyclodextrin derivatives as chiral selectors for direct gas chromatographic separation of enantiomers in the essential oil, aroma and flavor fields. In: Journal of Chromatography A. 843, 1999, pp. 99-121; Retrieved online January 1, 2013.
- ↑ Modified sugar molecules destroy viruses , derstandard.at of January 30, 2020.
- ↑ Jones ST, Cagno V, Stellacci F, Tapparel C et al .: Modified cyclodextrins as broad-spectrum antivirals , in: Science Advances , Vol. 6, no.5, eaax9318 of January 29, 2020. doi: 10.1126 / sciadv. aax9318 (English).
Web links
- Martin Gröger, Eva Katharina Kretzer, Andreas Woyke: Cyclodextrins. ( Memento of March 3, 2012 in the Internet Archive ) University of Siegen, 2001. (Detailed overview of cyclodextrins; PDF, 1.52 MB)
- CTD, Inc, Cyclodextrin Resource ( February 1, 2011 memento on the Internet Archive )