Prochirality

Prochirality is a stereochemical term coined by Kenneth R. Hanson in 1966 . It describes the property of a planar molecule that carries three different functional groups or substituents, or of a non-chiral, tetrahedral molecule with at most two identical substituents. The planar compound can form a center of chirality through an addition reaction , the tetrahedral one in the case of substitution or in the isomerization reaction of one of the identical substituents by a new substituent .
Stereochemical explanation
General
A prochiral connection has a plane of symmetry that divides the connection into two parts. The two sides are called enantiotopic . Depending on which side the attack is coming from, one or the other of the two mirror images is created. If none of the sides is attacked preferentially during the reaction, a racemic 1: 1 mixture of the respective stereoisomers is formed.
If there is also a chiral center in a prochiral compound, the compound is broken down into two diastereotopic parts. Again one or the other diastereomer is formed, depending on which side the attack is from. Even if a prochiral molecule is bound to a stereochemically differentiating surface or a receptor protein, the two sides of a planar or the identical substituents of a tetrahedral molecule are no longer stereochemically equivalent.
Addition to a planar molecule with a double bond
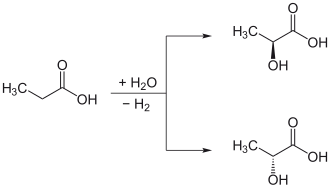
The trigonal-planar sp 2 -hybridized carbon atom of an organic compound can be converted into a chiral, now sp 3 -hybridized carbon atom in one step by adding HCl , Cl 2 , H 2 etc. The new substituent can occur either above or below the molecular level. The sp 2 -hybridized, planar substituted carbon atom is then called prochiral . The side from which the connection appears ( R ) -configured is called Re ; the other side, from which the connection appears ( S ) -configured, is denoted by Si . An attack from the Re or Si side does not mean that the product is ( R ) or ( S ) -configured, rather this depends on the priority of the substituents present and the priority of the added substance . One example is the addition of hydrogen to pyruvic acid , which creates chiral lactic acid .
Replacement of a substituent (stereo descriptors)
It is not necessary that the prochiral atom be sp 2 hybridized; it can also be sp 3 -hybridized and carry two identical substituents (stereoheterotopic groups), which means that this molecule is also not chiral. In the case of such compounds, the terms pro- ( R ) and pro- ( S ) are used:
The one of the two identical substituents that is replaced (initially only in thought) is arbitrarily assigned the higher CIP priority over its stereoheterotopic partner. All other priorities of the substituents with one another remain otherwise unaffected. If an ( S ) -configured “product” results in this way (mentally) , the substituent (or group) is called “pro- ( S )”; and the other is labeled “pro- ( R )”.
Because of this procedure, it is possible that an ( R ) product is formed when the pro- ( S ) -substituent is substituted .
For example, by replacing one of the two stereoheterotopic α-hydrogen atoms (hydrogen atoms of the methylene group , −CH 2 -) with an OH group, propionic acid is converted into chiral lactic acid . Replacing the pro- ( S ) -hydrogen atom of propionic acid leads to ( S ) -lactic acid. In this case, the above-mentioned rule that the priorities of the other substituents must not change is violated. However, this happens twice as the introduced hydroxyl group has a higher priority than the carboxy group as well as the methyl group according to CIP , so that the injuries cancel each other out and the ( S ) -product is created by substituting the pro- ( S ) -hydrogen atom . However, one replaces the same atom by an ethyl group , only occurs a violation of the rule on, and there is the corresponding ( R ) product (in this case 2-methylbutanoic acid).
literature
- Klaus Schwetlick: Organikum , Wiley-VCH, 2004, 22., completely revised a. updated edition, ISBN 978-3-527-31148-4 .
Individual evidence
- ↑ Kenneth R. Hanson: Applications of the Sequence Rule. I. Naming the Paired Ligands g, g at a Tetrahedral Atom Xggij. II. Naming the Two Faces of a Trigonal Atom Yghi in: J. Am. Chem. Soc. 88, 2731 (1966) .
- ↑ Stuart Cantrill: A Photo Finish , Nature Chem., 4, 5 (2012).
- ↑ PK Hashim, Nobuyuki Tamaoki: Induction of Point Chirality by E / Z Photoisomerization , Angew. Chem. Int. Ed., 50, 11729 (2011).
- ↑ Hermann J. Roth, Christa E. Müller, Gerd Folkers: Stereochemie und Arzneimittel , Wissenschaftliche Verlagsgesellschaft Stuttgart, 1998, pp. 261-262, ISBN 3-8047-1485-4 .
- ↑ a b University of Erlangen : Prochirality
Web links
- Entry to prochirality . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.P04859 Version: 2.3.